Safety of natural peptide bioregulators
Edited by Prof. Vladimir Kh. Khavinson,
Associate Member of the Russian Academy of Medical Sciences
Written by G.A. Ryzhak
Safety of Natural Peptide Bioregulators. – St. Petersburg: IKF Foliant, 2002. – 20 p.
The proposed scientific publication presents the results of a detailed study on peptide bioregulators isolated from animal organs and tissues and on the risk of their contamination with infectious agents, functionally active protooncogenes, nucleic acids, and prion proteins. The medical application of this class of pharmaceuticals has been proven entirely safe.
Introduction
The contemporary epoch is marked by an extraordinary diversity of unfavourable factors affecting the human organism: natural conditions, unbalanced nutrition, environmental factors, external damaging agents including ionising and microwave radiation and toxic substances. It results in the depletion of adaptation and compensation mechanisms, occurrence of various diseases and pathologic states, and, finally, in premature ageing.
The above problems necessitate the development and clinical implementation of new effective therapeutic agents and the methods of functional correction, intensification of resistance to adverse factors, inhibition of ageing, and prolongation of life span.
Long-term experience of applying peptide bioregulators extracted from the organs and tissues of young animals has confirmed the high efficacy of this class of substances in various diseases and pathologic states including those not responsive to the treatment with other medications.
However, the active application of animal-derived peptide bioregulators in medicine requires special control over the quality and safety of these substances, since raw material used in their production can contain components hazardous for the human organism. In this view, it becomes ever more significant to devise technologies securing the safety of such substances and to work out effective control methods proving the absence of infectious agents, protooncogenes, prion proteins or other objectionable components in them.
natural peptide bioregulators
The concept of bioregulation therapy based on the pathogenetic application of peptide bioregulators in various diseases, pathologic states, and ageing has been proposed and grounded in the course of 30-years’ research conducted at the Military Medical Academy (St. Petersburg) and St. Petersburg Institute of Bioregulation and Gerontology. Vyacheslav Morozov and Vladimir Khavinson were the first to isolate peptide bioregulators of multicellular systems from the hypothalamus, epiphysis, and vessel walls in 1971 [7]. These substances have been brand-named cytomedins.
The technology of obtaining cytomedins includes acetic extraction of polypeptide fractions from cattle and pig tissues, their precipitation and subsequent multistage purification. The final product is in the form of lyophilised powder for injection solutions.
These developments have enabled the creation of new pharmaceuticals – peptide bioregulators, which form a group of pharmacologically active substances with molecular weight within 1-10 kDa. Their administration to the organism results in the functional restoration of the very organs, which the cytomedins have been isolated from.
The Institute possesses 15 pharmaceuticals. All of them have undergone thorough trials. Some cytomedins have been approved for clinical use over 15 years before (Epithalamin® – the endocrine system bioregulator, Thymalin® – the thymus bioregulator, and Prostatilen® – the prostate bioregulator) [5]. In 1999, the Russian Ministry of Health certified the clinical application of Cortexin® (the cerebral cortex bioregulator) and Retinalamin® (the retina bioregulator) [15, 4]. Other bioregulators are undergoing various stages of clinical and experimental studies.
The technology of bioregulators manufacture and the methods of treatment with their use are covered by 70 licenses and USSR, Russian, and foreign patents. The majority of the developments have no analogues in the world.
Long-term experience of applying peptide bioregulators in healthcare has revealed their high effectiveness in various diseases and pathologic states including those not responsive to the treatment by other therapeutic means.
A new class of parapharmaceuticals – cytamins – has been created to expand the sphere for the application of bioregulators. Cytamins are balanced tissue-specific nucleoprotein complexes isolated from animal organs and tissues [11].
Today, 17 Cytamins are produced: extracted from the brain, liver, prostate, heart, thymus, bronchi, cartilages, pancreas, vessels, stomach, testes, epiphysis, thyroid, adrenal glands, kidneys, ovaries, and eye tissues.
Cytamins exert a targeted (organotropic) effect immediately upon the organs and tissues they have been isolated from. Being not drugs per se they produce a mild physiological regulatory influence on various functional systems of the organism, which enables their use as natural adaptogenes. Cytamins promote optimal functioning and full-value nourishment of organs and tissues, they do not contain any conservatives or other alien substances, produce no side effects, have no contraindications, and are compatible with any other nutrients and medications.
The patented technology of cytamins manufacture includes alkaline hydrolysis from tissue cells, consecutive precipitation of nucleoprotein complexes, their purification from ballast substances, and manufacture of the ready form as enterosoluble tablets or capsules.
Thus, two new classes of peptide bioregulators have been developed: pharmaceuticals cytomedins and parapharmaceuticals cytamins applied on a wide scale in medical practice to prevent various disease and pathologic states and treat for them.
safety of cytomedins and cytamins
Historical background of the prion diseases concept
The history of the prion diseases concept is set forth in detail in monograph by V.A. Zuev et al. “Prion diseases in humans and animals” [16]. Below we resort to some excerpts from this book.
The problem of prion diseases arose within the framework of the slow developing infections theory on the basis of the results of long-term investigations on mass diseases in sheep imported to Iceland from Germany in 1933. Despite some obvious clinical differences and uneven localisation of lesions in the animals’ organs and tissues, a principal similarity was revealed. This similarity can be summed up in four major signs of slow developing infections:
- unusually prolonged incubation period (for months and even years);
- slowly progressing course of the disease;
- untypical character of lesions;
- Inevitable lethal outcome.
Among the studied sheep pathologies, scrapie was investigated. This disease of sheep and goats was well known in many countries.
Three years later, in a geographically opposite region – New Guinea – a new disease, now known as kuru, was revealed among Papua cannibals and described. Its mass development pointed at its infectious nature.
These and a number of other diseases were united by a common typical symptom. All of them were manifested only as lesions to the central nervous system: a typical picture of so-called spongiform transformation of the grey and/or white brain substances and, sometimes, of the spinal marrow based upon primary degenerative processes (without inflammation signs) and, in some cases, accompanied by the formation of amyloid plaques and expressed gliosis.
This peculiarity of the pathomorphologic picture marked out the name for the entire group of diseases – transmissible spongiform encephalopathies. Their pathognomonic feature consisted in the transmissibility of spongiform alterations solely within the central nervous system.
For decades, any attempts to discover pathogenes of these diseases ended in a failure though their infectious nature was convincingly confirmed. Yet, in early 80s Stanley Prusiner, an American biochemist from the California University, claimed that this pathogene was a nucleon-free low molecular protein (27-30 kDa), which he named “the infectious prion protein” [13]. Prusiner proposed the term “prion” – an anagram from “proteinaceous infectious (particle)” as an infectious unit consisting of the infectious prion protein molecules. Prusiner was awarded a Nobel Prize in Biology or Medicine in 1997 for his prolonged investigations on slow developing prion infections.
Results of the studies carried out over the recent 15 years confirmed the prion nature of transmissible spongiform encephalopathy pathogenes completely. Consequently, these diseases were defined as prion ones.
The non-infectious (cellular) prion protein is vitally important. It is found in all mammals including humans. Among its distinguishing features is its high sensitivity to the digestive activity of protease K, which destroys this protein.
The infectious prion protein is preserved after the digestive impact of protease K. Its molecular weight equals 27-30 kDa.
The mechanism of accumulating the infectious prion protein in an infected organism has not been defined by now. At the same time, it is obvious that in a healthy organism the infectious prion protein entails the transformation of the normal prion protein into its infectious form due to the conformation changes of the normal prion protein. Therefore, the infectious prion protein is accumulated not via de novo synthesis of its molecules in an infected organism, but due to the conformation alterations of already synthesised normal molecules of a prion protein under the effect of the infectious prion protein.
The modern classification of prion diseases distinguishes human and animal pathologies and includes four human and six animal ones.
The list of human prion pathologies starts with Creutzfeldt-Jacob disease – the main in this group, while kuru and Herstmann-Streussler-Scheinker syndrome are considered to be its variants.
Scrapie is the main animal prion disease viewed as the prototype for all human and animal prion diseases.
In 1986, the United Kingdom was struck by an epizootic of bovine spongiform encephalopathy. As it turned out, the infectious prion protein originated from meat/bone flour. The technological process of its manufacture in early 80s implied a considerably reduced process of carbohydrate dissolving extraction of fat from bones, pluck, and heads of caws and sheep. This technological modification enabled the preservation of the infectious prion protein in raw material and caused mass contamination of ruminants after adding meat/bone flour to their forage as a protein supplement.
Started with two cases in 1986, the epizootic developed rather intensively and reached the peak of 1,000 cases in caws in 1992. Then the rate of new occurrences decreased notably and progressively.
Over this period, 23 sporadic cases of the so-called the new variant of Creutzfeldt-Jacob disease were registered, chiefly in the United Kingdom. The disease broke out among young patients, which was untypical.
Creutzfeldt-Jacob’s is a rare and fatal disease of worldwide distribution. Its total annual incidence in different regions is nearly equal and makes 0.3-1 case per 1,000,000 people. There has been revealed no correlation between Creutzfeldt-Jacob disease and the development of scrapie as the most frequent animal prion pathology. Creutzfeldt-Jacob disease has the same incidence in the scrapie-endemic United Kingdom and in Australia and New Zealand where scrapie has not been registered for years.
Wide-scale research was conducted in the United Kingdom in response to the epizootic of bovine spongiform encephalopathy in this country. Results of these investigations revealed the principal risk of infecting humans with prions from contaminated animals. It must be noted that the epizootic did not influence the total incidence of Creutzfeldt-Jacob disease in the United Kingdom as compared to other countries where no cases of the pathology in cattle were observed. Only the young age of all patients with the new variant of Creutzfeldt-Jacob disease attracts attention: all these people were less than 40 years of age. At the same time, young patients with this diseases constituted as little as 2 % of the total population (usually it was registered in the older age group, its peak striking the 60-65-year-olds).
Consequently, natural raw material of animal origin can potentially include infectious agents, protooncogenes, and nucleic acids. That is why, in the manufacture of preparations from animal organs and tissues a special attention is to be given to the purification of the active substance from objectionable admixtures and, first of all, to the infectious safety of such preparations.
Technological aspects of peptide bioregulators safety
In the manufacture of peptide bioregulators only the Russian-raised cattle is employed – calves and pigs under 12 months of age from the regions and farms where no human-endangering infectious diseases including transmissive bovine spongiform encephalopathy has been registered. It is to emphasise that Russia is known for its epizootological and epidemiological safety in respect to prion diseases.
The technology of obtaining cytomedins implies the acetic extraction of polypeptide fractions from calf and pig tissues, their precipitation and subsequent multistage purification until the outcome of active fractions with the molecular weight of 1-10 kDa. The final product is in the form of lyophilised powder intended for injection solutions.
The technology of extracting Cytamins includes alkaline hydrolysis of tissue cells, subsequent precipitation of nucleic complexes and their purification from ballast substances, drying of the half-product and manufacture of the ready form as enterosoluble tablets or capsules.
Thus, the patented methods of obtaining cytomedins and cytamins secure the maximal protection of these preparations from the interference of infectious agents. The active substance is extracted from animal organs and tissues at 3-7°C, pH 3.0 (cytomedins) or 11.0 (cytamins) for 6 days. These conditions are sufficient to fully inactivate all microbes and viruses even if no extra impacts are applied [8, 10].
Analysis of peptide bioregulators molecular weight
Molecular weight of cytomedins was studied by two methods – gel chromatography in Sefadex G-25 and electrophoresis in 15 % polyacrylamide gel. The results of gel chromatography revealed one peak in each chromatograms of the Cytomedins in the region of molecular weight under 10 kDa (Figures 1, 2, 3).
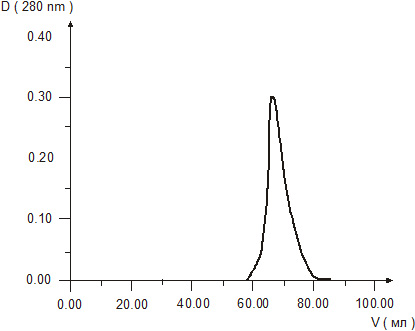
Figure 1. Gel chromatography of Cortexin solution.
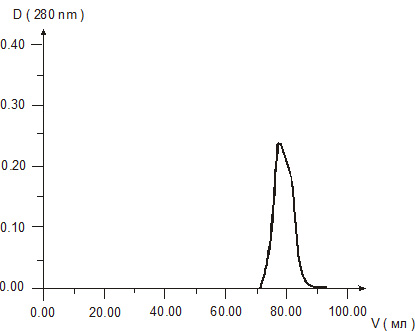
Figure 2. Gel chromatography of Retinalamin solution.
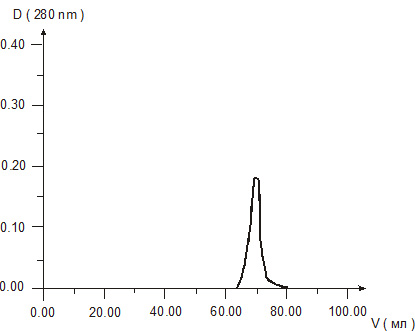
Figure 3. Gel chromatography of Epithalamin solution.
Electrophoresis in 15 % polyacrylamide gel demonstrated that Cortexin, Retinalamin, and Epithalamin had only one band each at the level under 10 kDa (Figures 4, 5, 6).
Figure 4. Electrophoresis of Cortexin solution in 15 % polyacrylamide gel.
Tracks 1, 5 – a standard solution of marker proteins;
Tracks 2, 3, 4 – Cortexin solutions in 3 series.
Figure 5. Electrophoresis of Retinalamin solution in 15 % polyacrylamide gel.
Tracks 1, 3 – a standard solution of marker proteins;
Track 2 – Retinalamin solution.
Figure 6. Electrophoresis of Epithalamin solution in 15 % polyacrylamide gel.
Tracks 1, 3, 5 – a standard solution of marker proteins;
Tracks 2, 4 – Epithalamin solutions in 2 series.
Table
Molecular weights of cytamins components (nucleoprotein complexes)
|
Cytamins |
Average molecular weight of macromolecular fractions, kDa |
Average molecular weight of low molecular fractions, kDa |
| Hepatamin |
200 |
5.0 |
| Vasalamin |
180 |
4.5 |
| Chondramin |
190 |
4.1 |
| Bronchalamin |
187 |
5.0 |
| Prostalamin |
208 |
5.0 |
| Testalamin |
186 |
4.8 |
| Epiphamin |
224 |
16.2 |
| Thymusamin |
250 |
15.8 |
| Cerebramin |
220 |
8.0 |
| Coramin |
190 |
3.2 |
| Ventramin |
224 |
4.5 |
| Pancramin |
224 |
4.6 |
Gel chromatography of Cytamins component analysis revealed that the nucleoprotein complexes (NPC) consisted of two groups of molecular associates (see Table). The molecular weight of the components of Cytamin fractions isolated from the tissues of similar origin demonstrated a close correlation.
The molecular weight of NPC macromolecular fractions ranged from 180 to 250 kDa, while those of low molecular fractions – within 3 to 10 kDa (except the thymic and epiphyseal bioregulators) Figure 7 shows gel chromatography curves for some cytamins. Their analysis demonstrates that almost all the bioregulators (except Coramin) contain a rather high amount of macromolecular components. The sharpness of the outcome peaks evidences the presence of a little quantity of substances of various molecular weights in the macromolecular fraction. Low molecular fractions are marked by a broad distribution by their molecular weight.
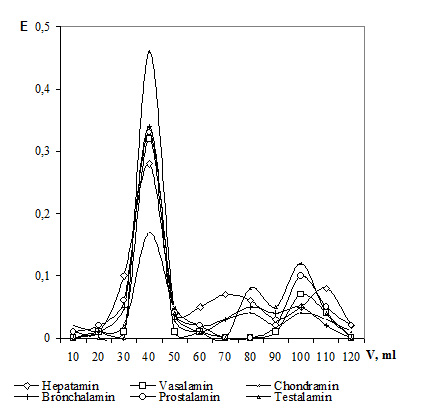
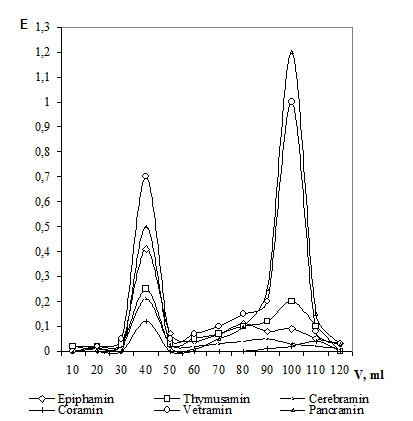
Figure 7. Gel chromatography of cytamins.
Assessment of the risk of contaminating peptide bioregulators with nucleic acids
The contamination of Cytomedins and Cytamins with nucleic acids was assessed by phenol-detergent extraction of nucleic acids from the bioregulators with their subsequent electrophoresis in 1 % agarose gel in a tris-acetate buffer (ethidium bromide staining) [10].
The results of the investigation displayed in Figure 8 confirmed the absence of nucleic acids in Cortexin and Retinalamin. This conformed to the analysis of the method of obtaining the bioregulators: the stage of ultrafiltration purification in hollow fibres with the limiting capacity of 15 kDa guarantied the deterrence of the bulk of nucleic acids, i.e. particles including over 45 nucleotides for single-chain molecules and over 23 nucleotides – for double-chain molecules. If supposedly, a very insignificant number of nucleic acids with molecular weight under 15 kDa and at concentrations indefinable by this method were present in the bioregulators, such DNA or RNA (within 45 nucleotides) could not have any matrix or infectious activity [8, 10, 1, 2].
Figure 8. Electrophoresis of Cytomedins in 1 % agarose gel after phenol-detergent extraction
Track 1 – molecular weight marker – bacteriophage lambda DNA treated with restriction endonuclease Hind III;
Track 2 – native DNA isolated from human blood lymphocytes (positive control);
Track 3 – Cortexin;
Track 4 – Retinalamin
The absence of luminescent material during ethidium bromide staining evidenced the lack of nucleic acids in bioregulators Cortexin and Retinalamin.
Cytamins were examined by the same method. The obtained results are exhibited in Figures 9 and 10. In Figure 9, the last tracks on the right and on the left display bacteriophage lambda DNA (molecular weight marker) treated with endonuclease Hind III. The second track on the left contains native DNA isolated by phenol-detergent method from human stomach mucous membrane (surgical material). Electrophoregram shows native human DNA as a discrete stripe above the bacteriophage lambda DNA fragment with the molecular weight of 23,000 pairs of bases. Tracks 3-14 contain the samples of 12 Cytamins isolated from various animal organs and tissues. As it is shown in the Figure, these samples appear as trains with the molecular weight from 0 to 23,000 pairs of bases.
Figure 10 demonstrates the results of treating the nucleic acids of Cytamins with nuclease S1 (Fermentas). Tracks 1, 3, 5, and 7 contain the untreated Cytamin samples, while Tracks 2, 4, 6, and 8 – nuclease S1 treated ones. The Figure reveals the reduced intensity of the trains in all the nuclease-treated samples below the fragment of bacteriophage lambda DNA with the molecular weight of 2,000 pairs of bases. This proves that DNA with the molecular weight under 23,000 pairs of bases contains a significant number of single-chain sites along its entire length or is presented in a single-chain form.
Figure 9. Electrophoresis of Cytamins in 1 % agarose gel
Figure 10. Electrophoresis of Cytamins in 1 % agarose gel before and after nuclease S1 treatment
The obtained results suggest that nucleic acids detected in Cytamins are the products of deep degradation of DNA and RNA, which can have no matrix or infectious activity.
Consequently, the analysis of the technology of obtaining Cytomedins and Cytamins from various animal organs and tissues and the experimentally confirmed degradation of nucleic acids exclude any risk of viable viruses or functionally active protooncogenes to be present in the investigated bioregulators [9].
Investigation of the protein components of Cytomedins and Cytamins
The World Health Organisation (WHO) recommends histochemistry, electron microscopy, electrophoresis, and immunoblotting with antibodies to PrP-protein for the analysis of biological material for prion proteins [6, 3, 12, 14].
To confirm the absence of prion proteins in Cytomedins and Cytamins their samples were examined at 200-fold concentration.
Electrophoresis in polyacrylamide gel was used for the preliminary assessment of protein content in Cytomedins. This method enabled the exposure or exclusion of proteins with molecular weight characteristic for prions.
As Figures 4, 5 and 6 demonstrated, no macromolecular proteins including prions were revealed in Cytomedins by electrophoresis.
For primary screening for prion infections the WHO recommends to use the histochemical method based on the Congo red staining of a studied sample [3, 14]. Characteristic luminescence in the crossed polarisers of a Congo-stained sample points at the presence of amyloid, i.e. the class of proteins including prions.
Since Cytomedins and Cytamins are water-soluble substances, the fixation of samples was performed not on a microscope slide but in polyacrylamide gel. The examined material was fixed in alcohol on a microscope slide and stained with 1 % water Congo red solution for 3 hours according to the standard protocol. Afterwards, the samples were treated with 1 % potassium iodide solution for 60 sec and washed with 70 % alcohol from the unbound stain three times for 10 min. The samples were examined in a dark field under cross polarisers at x300-x600. Histological samples of human amyloid liver were used as the positive control.
The results of the histochemical investigation are displayed in Figures 11, 12.
No selective binding of the stain was observed during Congo red staining of a Cortexin concentrated fraction fixed in polyacrylamide gel. The examination of the stained sample did not reveal any luminescence typical of amyloid aggregations either (Figure 11).
The sample of human amyloid liver gave bright selective staining with Congo red and emitted typical yellow-green luminescence of amyloid fibrils in crossed polarisers (Figure 12).
Thus, histochemically, no amyloid or amyloid-like protein compounds including prions were exposed in Cortexin. The same results were obtained for Retinalamin®, Epithalamin®, and Cytamins.
Figure 11. Histochemical analysis of Cortexin in polyacrylamide gel. Congo red staining, x600
No luminescence of amyloid is registered. The dots are bubbles in the gel; the pale spot is the dispersion of light in the gel.
Figure 12. Histochemical analysis of human amyloid liver, x600
Luminescence of amyloid fibrils in the crossed polarisers of the Congo-stained sample is observed.
By now, more specific and sensitive methods of prion exposure apart from histochemical Congo red staining have been worked out, for example, immunoblotting and direct electron microscopic exposure of prions in the form of scrapie-associated fibrils (SAF) combined with proteolysis.
Immunoblotting is a highly specific method for the exposure of various PrP-protein modifications including prions in a sample.
In this method monoclonal antibodies to PrPC and PrPSc 6H4 were employed (check-kit PRIONICS). The tested samples were homogenised in a sarcosyl buffer up to 10 % concentration and denatured in a SDS-mercaptoethanol buffer. Afterwards, 10 µl of each sample were applied to the surface of polyacrylamide gel in a cassette (Mini Protean II, Biorad). Electrophoresis was carried out for 2 hours at 80 V. One of the obtained laminae was stained according to Coumassie, while the tested samples from the other were electronically transferred to a high-capacity synthetic membrane PVDF for 10 hours at 40 V and 4°C (Transfer tank, Biorad). The protein was spread along the membrane with acetic acid solution “PonceauS” for 2 min, then the membrane was placed in a BLOCK buffer (check-kit PRIONICS) for an hour at room temperature. Incubation with monoclonal antibodies 6H4 (1:2,500) was carried out for 20 hours at 4°C. After the incubation with the second (goat-antimouse) antibodies the signal was exposed by chemoluminescent substrate NTB/BCIP.
The results of the investigation are displayed in Figure 13.
No binding of the antibodies with Cortexin and Retinalamin was observed. Consequently, the normal cellular prion protein (PrPC), which had definitely been present in the raw material, was effectively eliminated in the technological process and was not detected in the final product. No abnormal modifications of prion protein (PrPSc), i.e. prions, were revealed either.
Figure 13. Immunoblotting analysis of Cortexin and Retinalamin with antibodies to PrP-proteins
Tracks 1, 2 – Retinalamin® at the concentrations of 5 and 10 mg/ml, correspondingly;
Tracks 3, 4 – Cortexin at the concentrations of 5 and 10 mg/ml, correspondingly;
Track 5 – a standard solution of marker proteins.
Cytamins – Cerebramin®, Epiphamin, Ophthalamin®, and Chondramin® – were also tested by immunoblotting with antibodies 6H4 (check-kit PRIONICS).
Electrophoresis in polyacrylamide gel (Figure 14) did not reveal any proteins with molecular weight under 45-50 kDa in Chondramin, which confirmed the absence of prion proteins (27-30 kDa) in this bioregulator. No bands of binding, i.e. prion proteins, were exposed in Chondramin and Ophthalamin after incubating them on a PVDF membrane with antibodies 6H4 and subsequent staining (the samples were not treated with protease) (Figure 15).
Cerebramin and Epiphamin revealed weak signals within 30-35 kDa before proteolytic treatment, which pointed at the presence of normal cellular protein PrPC in the tested samples. After their proteinase K treatment, incubation with antibodies 6H4, and staining none of the tested bioregulators revealed a signal of binding with the antibodies (Figure 15).
Thus, immunoblotting demonstrated the absence of infectious prion proteins in Cytamins.
Figure 14. Analysis of Cytamins by electrophoresis in polyacrylamide gel
Track 1 – Cerebramin®
Track 2 – Chondramin®
Track 3 – Ophthalamin®
Track 4 – Epiphamin®
Track 5 – marker proteins (molecular weight 30, 40 kDa).
Figure 15. Analysis of Cytamins by immunoblotting
Track 1 – Cerebramin®
Track 2 – Chondramin®
Track 3 – Ophthalamin®
Track 4 – Epiphamin®
Left-side tracks – before proteinase K treatment;
Right-side tracks – after proteinase K treatment.
Direct electron microscopic exposure of prions in the form of scrapie-associated fibrils combined with proteolysis detects prions even by two of their characteristic features – proteolytic resistance and morphology of fibril structures formed by prions.
Some authors point at the ability of separate prion molecules to aggregate and form plait structures of SAF type already after the treatment with low concentrations of proteinase K. That is why, in some cases (obviously, in heavily glycolysed forms) prions cannot be detected by electron microscopy before proteolytic treatment.
To enhance the sensitivity of electron microscopy, an aggregated protein fraction in the tested samples was concentrated by three-fold freezing/thawing of a saturated bioregulators solution. Then, 5 ml of the solution of each of the tested bioregulators were centrifuged for 2 hours at 120,000 g in ultracentrifuge L-8 (Beckman, USA). The obtained precipitate was dissolved in
50 µl of bidistilled water to attain an approximately 200-fold concentration of the fractions, which could contain prions. Concentrated precipitates of the aggregated proteins of Cytomedins and Cytamins were applied to formvar-carbon bases (sorption time – 10 min) and examined by electron microscopy at x30,000-100,000 (JEM-100S, JEOL, Japan).
Afterwards, the bases with the sorbed samples were treated with proteinase K for 20 min at 0.2 mg/ml, 37°C. The bases were contrasted with 1.5 % solution of sodium phosphor-wolfram acid and examined again in the microscope for proteolytically resistant SAF including prions. The prions of yeast, similar in t