NAD for Longer Lifespans
To live well and flourish as human beings, we need lots of nicotinamide adenine dinucleotide (NAD), a nicotinic-based molecule found within nearly every cell in the human body. However, as we age, we lose this all-important molecule largely from the decline of proper sirtuin activity.[1]
The longevity genes, called ‘sirtuins’, are named after the yeast SIR2 gene, the first sirtuin to be discovered. Sirtuins are NAD-utilizing enzymes and much research has been done into how the body controls sirtuins. Sirtuins are thought to be a primary reason our bodies develop diseases when we are old, but not when we are young.
Sirtuins: NAD-Utilizing Enzymes
| LEXICON
AlphaTOR inhibitor—a statin AMPK (AMP-activated protein kinase) is known as a master regulator of metabolism. Exercise is the application of stress to our bodies. Fisulin is the first SIRT1-activating compound, or STAC, was a polyphenol. Flourish improves and make up the loss due to aging. FOXO3 is a variant has been shown to related to longevity in humans. NAMPT is the gene in our bodies that recycles NAD. NAD (nicotinamide adenine dinucleotide) is used by over five hundred different enzymes and its loss results in the decline in sirtuin activity. Nicotinamide riboside (NR) is converted to NMN. Nicotinamide mononucleotide (NMN) converts to NAD. Pyrazinamidase/ nicotinamidase 1 (PNC1) boosts NAD production. Resveratrol is a type of natural phenol, produced in certain plants when under attack by pathogens, such as bacteria or fungi. Sirtuin genes are named after the yeast SIR2 gene, the first one to be discovered. |
Shin-ichiro Imai, an associate of Dr. David Sinclair when he was at MIT, and now a professor at Washington University, discovered much of the valuable material about sirtuins. Principally, that they are NAD-utilizing enzymes. He also does research into how the body controls sirtuins.
David Sinclair, PhD, AO is best known for his work on genes and small molecules that delay aging, including the sirtuin genes, resveratrol, and NAD precursors. He is the author of a new book on aging published in 2019 Lifespan: Why We Age and Why We Don’t Have To.
Restriction in Mice
Sinclair and his lab at Harvard discovered that when calories are restricted in mice, yeast lifespan is extended by turning up genes that recycle NAD, thereby giving the sirtuins a growth boost.[2]
Hidden within the sometimes-confusing way scientists talk about science are several repeating themes. Among these are low energy sensors transcription errors. Plus, additional NAD and sirtuin boosters, stress resistance, and longevity factors.
The mice in Sinclair’s study were 20 months old, roughly the equivalent of a 65-year-old human. The researchers had fed them a molecule intended to boost the levels of NAD, which they believed would increase the activity of sirtuins. If the mice were developing a ‘running addiction,’ that would be a very good sign.
Why? One of the key findings of a lab study, published in 2018, was that when treated with an NAD-boosting molecule that activated endothelial cells that line up when treated with an NAD-boosting molecule that activated the SIRT1 enzyme, the elderly mice’s endothelial cells, which line the blood vessels, were pushing their way into areas of the muscle that weren’t getting very much blood flow.[3]
Scientists still don’t know everything about why this happens. They don’t know what sort of molecules will work best for activating sirtuins or the correct dose. Hundreds of different NAD precursors have been synthesized, and there are clinical trials in progress to answer that question and more.
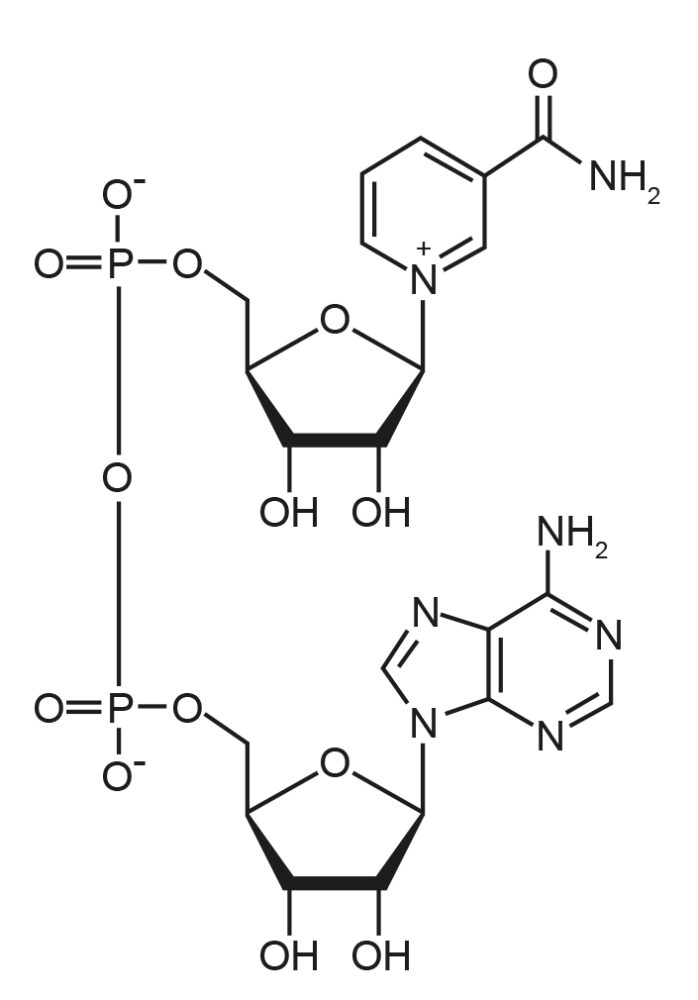
Figure 1: Dr. David Sinclair’s book: Why we age and why we don’t have to.
Exercise is the Application of Stress
Exercise, by definition, is the application of stress to our bodies. It raises NAD levels, which in turn activates the survival network, which turns up energy production and forces muscles to grow extra oxygen-carrying capillaries.
What happens on the other side of the thermostat? The picture is a bit less clear, but we have some promising leads from the laboratory yeast S. cerevisiae. A seemingly lowly, single-celled yeast with a sweet tooth (its name means ‘sugar-loving’) to its rightful place as one of the world’s most important research organisms.
We know from work in Sinclair’s lab that raising the temperature of yeast—from 30°C to 37°C, just below the limits of what those single-celled organisms can sustain—turns on the pyrazinamidase/ nicotinamidase 1 (PNC1) gene and boosts their NAD production, so their sirtuin proteins can work that much harder.[4]
What’s fascinating is not so much that these temperature-stressed cells lived 30 percent longer but that the mechanism is the same as that evoked by calorie restriction.
NAMPT, the Gene in our Bodies that Recycles NAD
Sauna studies show that temporary heat exposure may be good for us. But they do not tell us why. If yeast is any guide, more knowledge of NAMPT, the gene in our bodies that recycles NAD, may be useful.[5] NAMPT is turned on by a variety of adversity triggers, including fasting and exercise, which makes more NAD so the sirtuins can work hard at making us healthier.
Sinclair et al have never tested if NAMPT is turned on by heat, but that could be done by others. Either way, one thing is clear: it does us little good to spend our entire lives in the thermoneutral zone. Our genes didn’t evolve for a life of pampered comfort. A little stress to induce hormesis occasionally may go a long way.
But dealing with biological adversity is one thing. Over-whelming genetic damage is another. A bit of adversity or cellular stress is good for our epigenome because it stimulates our longevity genes.
If this sounds chaotic, it is. Nevertheless, we need this chaos for order to emerge. Without it, the molecules that must come together to sustain life would not find each other, and they would not fuse. The human sirtuin enzyme called SIRT1 serves as a good example. Precise vibrating sockets on SIRT1 simultaneously clasp onto an NAD molecule and the protein it wants to strip the acetyls from a histone or a special molecule. The two captured molecules immediately lock together, just before SIRT1 rips them apart in a different way, producing vitamin B3 and acetylated adenine ribose, waste products that are recycled back to NAD.
Metformin Impacts Many Diseases
Many life extension enthusiasts are now dosing with the diabetic drug metformin. The beauty of metformin is that it KOs many of the diseases of aging.
As does AMPK (AMP-activated protein kinase), a master regulator of metabolism. Cells activate AMPK when they are running low on energy, and AMPK is activated in tissues throughout the body following exercise or during calorie restriction.
AMPK makes more NAD and turns on sirtuins and other defenses that act against aging engaging the survival circuit upstream of these conditions, ostensibly slowing the loss of epigenetic information and keeping metabolism in check, so all organs stay younger and healthier.
When they are activated, either by low-calorie or low-amino-acid diets, or by exercise, organisms become healthier, disease resistant, and longer lived.
Figure 2: Graphic here of the process described above.
Tweaking Rapamycin, Metformin, Resveratrol, and NAD Pathways
Molecules that tweak these pathways, such as rapamycin, metformin, resveratrol, and NAD boosters can mimic the benefits of low-calorie diets and exercise and extend the lifespan of diverse organisms.[6]
By engaging our bodies’ survival mechanisms in the absence of real adversity, will we push our lifespans far beyond what we can today? And what will be the best way to do this?
Could it be a souped-up AMPK activator? An alphaTOR inhibitor? The first SIRT1-activating compound, or STAC, was a polyphenol called fisetin. Or a combination of them with intermittent fasting and high-intensity interval training? The potential permutations are virtually endless.
Another STAC is NAD, sometimes written as NAD+. NAD has an advantage over other STACs because it boosts the activity of all seven sirtuins. NAD was discovered in the early twentieth century as an alcoholic fermentation enhancer. That was unexpected: if it hadn’t had the potential to improve the way we make booze; scientists might not have been so charmed by it.
Curing the Canine Equivalent of Pellagra
Instead, they worked on it for decades, and in 1938 NAD was able to cure black tongue disease in dogs, the canine equivalent of pellagra. It turned out that NAD is a product of the vitamin niacin, a severe lack of which causes inflamed skin, diarrhea, dementia, skin sores, and ultimately death. And because NAD is used by over five hundred different enzymes, without any NAD, we’d be dead in thirty seconds.
By the 1960s, however, researchers had concluded that all the interesting research on NAD had been done. For decades to come, NAD was simply a housekeeping chemical that teenage biology students had to learn about—with all the enthusiasm of a teenager doing housekeeping. That all changed in the 1990s, when we began to realize that NAD wasn’t just keeping things running; it was a central regulator of many major biological processes, including aging and disease. That’s because Shin-ichiro Imai and Lenny Guarente showed that NAD acts as fuel for sirtuins.
Without sufficient NAD, the sirtuins don’t work efficiently: they can’t remove the acetyl groups from histones, they can’t silence genes, and they can’t extend lifespan. And we sure wouldn’t have seen the lifespan-extending impact of the activator resveratrol! Sinclair and others also noticed that NAD levels decrease with age throughout the body, in the brain, blood, muscle, immune cells, pancreas, skin, and even the endothelial cells that coat the inside of microscopic blood vessels. But because it’s so central to so many fundamental cellular processes, no researchers in the twentieth century had any interest in testing the effects of boosting levels of NAD.
“Bad stuff will happen if you mess with NAD,” they thought. But not even having tried to manipulate it, they didn’t really know what would happen if they did. The benefit of working with yeast, though, is that the worst-case scenario in any experiment is a yeast massacre. There was little risk in looking to boost NAD in yeast.
This’s why Sinclair’s lab members worked on this. The easiest way was to identify the genes that make NAD in yeast. They first discovered a gene called PNC1, which turns vitamin B3 into NAD. That led the lab to try boosting PNC1 by introducing four extra copies of it into the yeast cells, giving them five copies in total.
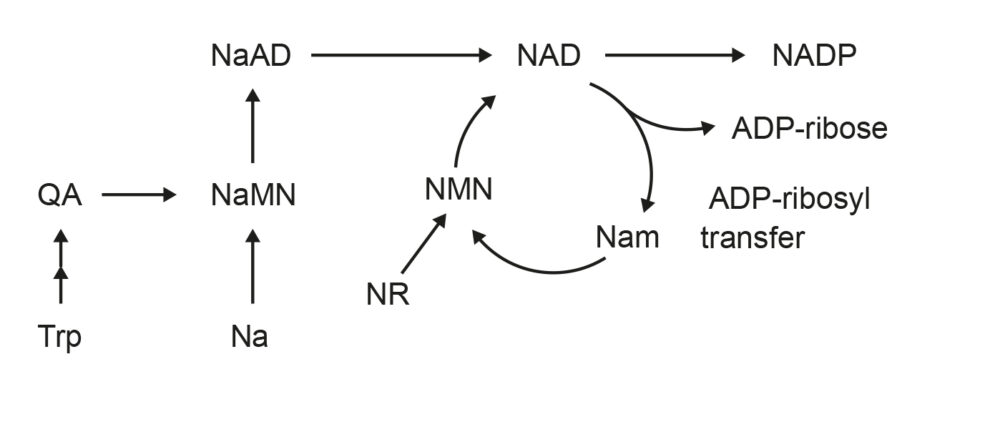
Figure 3: Graphic here of NAD cycle.
Living Longer with Sirtuins
Those yeast cells lived 50 percent longer than normal, but not if the lab removed the SIR2 gene. The cells were making extra NAD, and the sirtuin survival circuit was being engaged! Could Sinclair’s lab do this in humans? Theoretically, yes. They had already established that they had the technology to do this, using viruses to deliver the human equivalent of the PNC1 gene called NAMPT. But turning humans into transgenic organisms requires more paperwork and considerably more knowledge about safety—for the stakes are higher than a yeast massacre. That’s why the researchers once again began searching for safe molecules that would achieve the same result.
Another Route
Charles Brenner, who is now the head of biochemistry at the University of Iowa, discovered in 2004 that a form of vitamin B3 called nicotinamide riboside (NR), is a vital precursor of NAD.[7] He later found that NR, which is found in trace levels in milk, can extend the lifespan of yeast cells by boosting NAD and increasing the activity of Sir2. Once a rare chemical, NR is now sold by vast amounts each month as a nutraceutical.
In the body, NR is converted into NMN, which is then converted into NAD. Give an animal a drink with NR or NMN in it, and the levels of NAD in its body go up about 25 percent over the next couple of hours, about the same as if it had been fasting or exercising a great deal. Sinclair’s friend from the Guarente lab Shin-ichiro Imai demonstrated in 2011 that NMN could treat the symptoms of type-2 diabetes in old mice by restoring NAD levels. Then researchers in Sinclair’s lab at Harvard showed we could make the mitochondria in old mice function just like mitochondria in young mice after just a week of NMN injections.
Human studies with NAD boosters are ongoing. So far, there has been no toxicity, not even a hint of it. Studies to test its effectiveness in muscle and neurological diseases are in progress or about to begin, followed by super-NAD–boosting molecules that are a couple of years behind them in development. But a lot of people haven’t been content to wait for these studies, which can take years to play out. And that has given us some interesting leads about where these molecules, or ones like them, might take us.
NAD Boosters
We know that NAD boosters are an effective treatment for a wide variety of ailments in mice and that they extend their lifespan even when given late in life. We know that emerging research strongly suggests they could have a similar, if not duplicative, effect on human health. But a trial in 2018 to test whether an NAD booster could restore the fertility of old horses was successful, surprising the skeptical supervising veterinarian.
These anecdotal reports of restored menstruation and fertile horses are early but interesting indicators that NAD boosters might restore failing or failed ovaries. We also must remember what NMN does: it boosts NAD, and this boosts the activity of the SIRT2 enzyme, a human form of yeast Sir2 found in the cytoplasm.
All therefore are early indicators of restored ovarian function in humans, anecdotal as they may be, are so fascinating. If true, the mechanisms that work to prolong life bolster the case for NAD boosters. But the early indicators of the safety and effectiveness of the molecules in yeast, worms, and rodents are such that many people have already begun their own private human experiments.
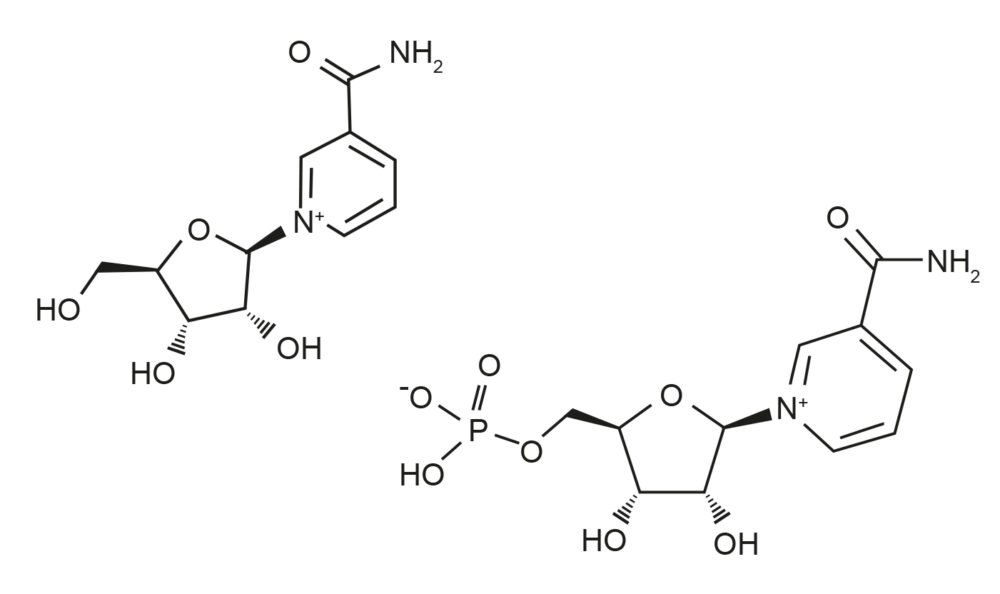
Figure 4: Graphic here of chemical difference between NAD, NR & NMN.
Come What May
By engaging our bodies’ survival mechanisms in the absence of real adversity, will we push our lifespans far beyond what we can today? And what will be the best way to do this? Could it be a souped-up AMPK activator? An alphaTOR inhibitor? The first SIRT1-activating compound, or STAC. Or a combination of them with intermittent fasting and high-intensity interval training? The potential permutations are virtually endless.
But as mice age, and possibly as we do as well, these sirtuins become scattered all over the genome, having been recruited away to repair DNA breaks elsewhere, and many of them never find their way home. This loss is exacerbated by a drop in NAD levels—the same thing we first saw in old yeast. Without sirtuins to spool the chromatin and silence the transposon DNA, cells start to transcribe these endogenous viruses.
Silencing the Jumping Genes
We may eventually find out that as NAD levels decline with age, sirtuins are rendered unable to silence retrotransposon DNA. Perhaps one day, safe antiretroviral drugs or NAD boosters will be used to keep these jumping genes silent.[8]
Today, many of Sinclair’s colleagues are just as optimistic, even though they will not openly admit it. I’d bet that about a third of them take metformin or an NAD booster. A few of them may even take doses of rapamycin intermittently.
Nicotinamide riboside, or NR, is converted to NMN, so whilst some people take NR instead of NMN, it is possibly not as effective as NMN. Studies are not always favorable to its use. Cheaper still are niacin and nicotinamide, but they don’t raise NAD levels as efficiently as NMN and NR do.
Some scientists have suggested NAD boosters could be taken with a compound that provides cells with methyl groups, such as trimethylglycine, also known as betaine or methylfolate.
Conceptually, this makes sense—the “N” in NR and NMN stands for nicotinamide, a version of vitamin B3 that the body methylates and excretes in urine when it is in excess.
And on top of what we already know about metformin, NAD boosters, rapalogs, and senolytics every day the odds increase. In time, even more effective molecule or gene therapy will no doubt be discovered, as brilliant researchers around the world join the global fight to treat aging, the mother of all diseases. In the meantime, we all seek out the best that is available today.
References
[1] Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010 Dec;35(12):669-75.
[2] Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016 Jun 14;23(6):1093-112.
[3] Das A, Huang GX, Bonkowsk MSi, et al. Impairment of an endothelial NAD+-H2S signaling network is a reversible cause of vascular aging. Cell. 2018 Mar 22; 173(1): 74–89.
[4] Anderson RM1, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003 May 8;423(6936):181-5.
[5] Shin-ichiro I, Guarente L. It takes two to tango: NAD+ and sirtuins in aging/longevity control. NPJ Aging Mech Dis. 2016;2:16017.
[6] Sabatini DM. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci U S A. 2017 Nov 7;114(45):11818-25.
[7] Trammell SA, Schmidt MS, Weidemann BJ, Redpath P, Jaksch F, Dellinger RW, Li Z, Abel ED, Migaud ME, Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat Commun. 2016 Oct 10;7:12948.
[8] De Cecco M1, Criscione SW, Peckham EJ, Hillenmeyer S, Hamm EA, Manivannan J, Peterson AL, Kreiling JA, Neretti N, Sedivy JM. Genomes of replicatively senescent cells undergo global epigenetic changes leading to gene silencing and activation of transposable elements. Aging Cell. 2013 Apr;12(2):247-56.
