ACF228: the Breakthrough Antidote to Aging
Everyday we see our aging bodies in the mirror reflecting hair loss, irregularly pigmented skin and wrinkled, lined, and sagging faces. Internally the body’s organs also undergo an age-related decline. Processes at the cellular level cause inadequate DNA repair and cell replication responsible for the deterioration of cells. This in turn leads to cell atrophy, loss of function, and most importantly, organ and muscle shrinkage. For example, the human brain loses a third of its weight between ages 35 and 70. Other organs such as the heart, liver, and kidneys, as well as muscles and vertebrae undergo a similar diminution.
Fortunately, many of our cells fall into the category of normally dividing; that is, they are continually replaced when they die, such as in the skin or hair. On the other hand, we are born with a fixed number of post-mitotic, or non-replicating cells, such as those in the central nervous system (CNS) and heart, which are not replaced upon their death. Some are lost daily in a relentless progression during an organism’s lifespan. When cell loss exceeds a critical level, organ failure results with subsequent death of the organism.
Prominent scientists have proposed many theories that explain the biological and chemical processes involved in “aging,” the vital loss of stem cells and cell function leading to cell death, followed by organ atrophy, and eventually death of the organism. One in particular, the Free Radical Theory of Aging, first proposed more than 50 years ago by Professor Denham Harman, MD, PhD, asserts that aging is largely a result of accumulative oxidative damage produced as a byproduct of aerobic cellular respiration. (1) This means that it may be dangerous to consume oxygen without adequate free-radical protection.
Prof. Harman concluded these facts after observing that ionizing radiation causes premature aging corresponding to an increase in the production of oxygen free-radicals.
Respiration occurs within our cells’ mitochondria when nutrients undergo an elaborate series of chemical reactions in the presence of oxygen to generate energy in the form of adenosine triphosphate (ATP), an essential ingredient to life. Mitochondria generate ATP as well as highly toxic and reactive oxygen free-radicals that subsequently damage cellular structures including membranes, proteins, and both nuclear and mitochondrial DNA. Simply stated, then, free radicals cause aging, or at least they play a significant role in the aging process. See Figure One.
Fig. One. Free radicals damage blood vessels leading to ischemia. This is reversible with radicals scavenger therapy.
During later years, esteemed scientists identified the mitochondria as the principal source of endogenous oxidants such as superoxide and hydroxyl anions, and hydrogen peroxide. (2) In the eighties, Prof. Harman associated cellular free-radical damage not just with aging, but with major age-related diseases, such as atherosclerosis, cancer, Alzheimer’s disease, and diabetes. (3) Since then, hundreds of studies link oxidative damage with degenerative diseases.
What can we do about it?
Consider the sobering fact that one of the most fundamental biological processes – respiration – that ensures our daily survival, day after day, decade after decade, eventually contributes to our demise.
But what can we do about it?
One determined scientist set out to answer that question. Using Harman’s Free Radical Theory of Aging as a springboard and equipped with an triple education in chemistry, physics, and medicine, Dr. Richard Lippman has spent over 30 years in a quest to study the mechanisms involved in aging itself, to quantify and monitor free radical damage at the cellular level, and to identify and formulate unique scavenger compounds capable of neutralizing oxidative assaults occurring 24/7 within our mitochondria.
The goal: to slow the rate of aging and extend lifespan.
Dr. Lippman has authored 27 papers on free-radical chemistry and medicine, and has invented several innovative medical products, including the nicotine patch. He was awarded US patent Nr. 4,695,590 entitled “Method for Retarding Aging,” and significantly, it is the only US anti-aging patent ever approved by the US Patent Office. It describes the oral and topical administration of specific compounds (known as hydroxy-substituted diphenylalkyl derivatives) that can accomplish the purpose set out in the patent’s title. In 1996, he was nominated for the Nobel Prize in Medicine for his anti-aging research. Through many years of research, he has developed a powerful free-radical scavenger formulation known as Aging Control Formula 228, or ACF228, which has been registered with the Swedish and Italian equivalents of the FDA.
Strongest Toxins on the Planet
According to Dr. Lippman, “Free radicals are the strongest toxins on the planet, a thousand times more destructive to living tissue than cyanide,” and “the likely culprits responsible for many aspects of aging due to their unstoppable 24/7 production.” He estimates that just by metabolizing oxygen, we are subject to approximately 7,000 free radical attacks daily, which come in the form of lethal, relentless cascades much like taking a barrel ride over Niagara falls.
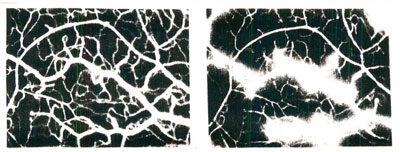
Fig. Two. Cascading free radicals cause many aspects of aging.
The key reactive oxygen “culprits” include singlet oxygen (an activated form of oxgen, O2′), hydrogen peroxide (H2O2), and the superoxide (O2¯?) and hydroxyl (OH?) free radicals. Superoxide, which is biologically noxious and remains active in the body’s cells longer than most other free radicals, has been implicated in the pathogenesis of many diseases. The most powerful of all radicals, the hydroxyl radical, has a much shorter nanosecond lifespan and wreaks havoc by causing, among other damage, genetic mutations and the formation of lipid peroxides, i.e. the products of free-radical attack upon lipids in cell membranes. Hydrogen peroxide and singlet oxygen also exhibit deleterious oxidative effects.
As a result of aerobic metabolism, most organisms possess certain anti-oxidative defenses, such as the superoxide scavenging enzyme, superoxide dismutase (SOD), which neutralizes superoxide, and the enzyme catalase, which scavenges the hydrogen peroxide formed when superoxide is quenched. However, as we will see below, the reactions involved in cellular respiration are not 100% efficient and our natural radical defense systems are somewhat limited. Also, under certain metabolic stressors, these defense systems can become overwhelmed and function even less efficiently. Add to that the fact that deactivating enzymes for hydroxyl free radical and singlet oxygen do not exist in nature. Everyday examples of these oxidative phenomena can be seen in the cracking of your windshield wipers’ rubber or the orange-peel appearance of your aging skin. See Figure Three.
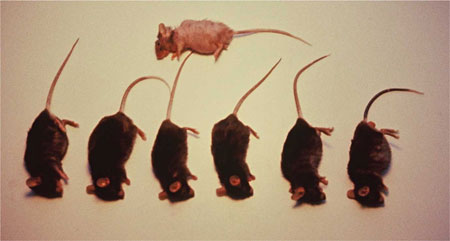
Fig. Three. Strong radical-scavenger fed mice (below) versus surviving control mouse (top).
Probing the Aging Process
In order to better understand the underlying mechanisms involved in the aging process, one of Dr. Lippman’s primary goals was to quantify and monitor free-radical activity in living cells, and use that knowledge to devise effective protocols for strengthening our defenses against oxidative damage.
He developed a technique using chemiluminescent probes which can enter the cells’ mitochondria and emit light in proportion to the concentration of various reactive oxygen species and radical scavengers. Note that chemiluminescence is the emission of light by an atom or molecule that is in an excited state. An example is the cold light produced by fireflies.
Dr. Lippman’s early experiments showed that many sulfur-containing compounds (for example, l-methionine and l-glutathione) could destroy carcinogenic nitroso compounds and other active oxygens. In subsequent work, a variety of substances were evaluated for their antioxidant potential by measuring free radicals and lipid peroxides in actively respiring mitochondria in human liver tissue using chemiluminsecent detection. Among these were the antioxidants BHA and BHT that Prof. Harman had demonstrated decades earlier extended the lifespan of mice by as much as 50 percent. Those compounds with the highest free-radical scavenging abilities were identified for use in potent formulations such as in ACF 228.
Dr. Lippman’s chemiluminescent probe experiments revealed several fascinating findings – that the chemical reactions involved in mitochondrial respiration are only 94 to 98% efficient in humans (with the remainder converted to byproducts), lower in other mammals, and only 84% efficient in bacteria. From an evolutionary standpoint, consider that humans utilize oxygen more efficiently than other mammals, and thereby generate comparatively fewer oxidation products, exhibit lower average body temperatures, and attain longer lifespans. But even so, extremely toxic byproducts are still generated. In a related finding, Dr. Lippman demonstrated that strong free-radical scavengers, by deactivating oxygen free-radicals, also lower the human body temperature from 98.6°F to approximately 97°C and raise metabolic efficiency to the higher end of the 94-98% range. The discovery of the “Temperature Lowering and Efficiency Effect” contributed to his Nobel Prize nomination.
Also essential to Dr. Lippman’s research was his invention of a non-invasive instrument that monitors and quantifies lipid peroxides in vivo using reflective near-infrared (IR) detection. Consequently, in time course studies Dr. Lippman determined free-radical activity and the quenching abilities of various scavengers in the bloodstreams of animals and humans (including his own!). In fact, using this technique allowed Dr. Lippman to perfect his tried-and-true formula, ACF 228, after having evaluated and discarded 227 previous versions.
In the course of the probe experiments and near-IR in vivo monitoring, Dr. Lippman found that only strong free-radical scavengers such as the sulfur-containing compounds l-methionine, l-acetyl cysteine, n-acetyl cysteine, and l-glutathione, as well as BHT, BHA and nordihydroguairetic acid (NDGA) can effectively neutralize active oxygens, including the ubiquitous superoxide and hydroxyl radicals, and singlet oxygen. On the other hand, weak antioxidants such as vitamins C and E showed only marginal effects. He also discovered that consuming these strong scavengers every six hours maintains sufficiently high blood levels to effectively destroy free radicals round the clock. This led to the “one pill/one meal regimen” of approximately 250 mg of a strong, potent, and effective free-radical scavenger, or combination thereof, such as ACF228, with each meal. Note that 250 mg is an adequate amount; Lippman confirmed Harmon’s earlier finding that mega-dosing with gram quantities of antioxidants not only does not work, but, in fact, creates the deleterious effect of increasing the rate of oxidation, and consequently, aging.
Dr. Lippman demonstrated that the “one pill/one meal” protocol reduces oxidative damage, increases mitochondrial efficiency, and slows aging at the cellular level, including the post-mitotic cells of the heart and central nervous system. In mice, it extends lifespan. For him, “taking one pill with every meal for the last three decades has been a slam dunk.”
ACF228: Comprehensive Anti-aging Cocktail
ACF228 is a multi-ingredient formula that combats the degenerative mechanisms involved in aging on many levels. In addition to four free-radical scavengers, it contains an anti-cross-linker (see definition below), a longevity -booster, a mitochondrial enhancer, a heavy metals chelator, a phytonutrient, and other targeted ingredients listed in boldface below.
N-acetylcysteine, l-methionine, BHT, and NDGA , as we have seen, are among the strong free-radical scavengers determined by Dr. Lippman to effectively neutralize oxygen free-radicals and peroxides. Note that NDGA is the ingredient extensively detailed in the patent, “Method for Retarding Aging,” in which several studies are presented. These include a double-blind human trial where NDGA was found to reduce levels of capillary lipid peroxides; in vitro studies, where NDGA increased ATP production in human brain cells and mitochondrial efficiency in human liver cells; and an animal study, in which older NDGA- supplemented mice retained their youthful, healthy appearance (with less skin wrinkling! See Figure Three) compared with non-medicated controls. ACF228 also contains the peroxide scavenging enzyme, catalase. The formulation contains optimal concentrations, i.e. approximately 250 mg of a combination, of strong free-radical scavengers to provide a synergistic effect against various levels of free-radical attack.
Partly as a result of free-radical damage, another process implicated in aging is glycation, i.e. the cross-linking of proteins and sugars to form non-functioning structures (amides) in the tissues of the body. This mechanism causes irreversible alterations of critical proteins and thus hinders normal cell function. In a pathological interplay, free-radical damage influences altered proteins, which in turn may further accelerate oxidative processes, leading to cellular damage at the DNA level. Glycation has been implicated in skin wrinkling and many degenerative diseases such as Alzheimer’s, cataract, cancer and heart disease. (4-7) The dipeptide carnosine not only inhibits oxidative damage but also interferes with the glycation process, (8-11) making it a critical anti-aging nutrient. A common example is found in the cross-linking of the lens of the eye that eventually leads to cataracts.
ACF228 is also formulated with the longevity booster, resveratrol, that has been shown to increase life span and enhance mitochondria in various species. (12-16) Found in grapes and red wine, resveratrol is a potent antioxidant (16) that enhances mitochondrial energy production (14) and positively influences gene expression similarly to calorie restriction, (13,15,17,18) the mechanism underling its life extending properties. Studies indicate that resveratrol may have therapeutic value in treating a broad range of disorders, including cardiovascular disease, cancer, arthritis, and Alzheimer’s disease. (19-21)
Diindolylmethane (DIM) is a cancer-fighting phytonutrient isolated from cruciferous vegetables, such as broccoli, cabbage, and watercress. It alters favorably estrogen metabolism and protect against hormone-dependent cancers (i.e. breast, cervix, and prostate) in both men and women. (22-24)
Dimercaptosuccinic acid (DMSA) is known as the premier heavy metal chelation compound since it crosses the blood-brain barrier to safely and effectively complex with and removes heavy metals. Only DMSA can extract unwanted metals from the brain such as lead and mercury. Due to the presence of these heavy metals in the environment and food chain, i.e. fish population, it detoxifies the body’s tissues and organs in order to avoid nerve damage and consequent slowed muscle and cognitive reactions. An everyday example of slowed reactions may well be seen in the driver ahead of you at a stop light who needs 3 to 5 seconds to react to a green light.
The formulation also contains the key minerals selenium, an important component of radical-scavenging enzymes, chromium polynicotinate, which supports healthy weight control and iodine, thought to stabilize hormone production, lower cancer risk, and improve thyroid function.
Chronological Age? Biological Age
With its broad spectrum of anti-aging benefits, ACF228 is an essential component of any anti-aging program. But to achieve maximum benefits, Dr. Lippman suggests that ACF228 be used in conjunction with (a) bioidentical hormone replacement, (b) superior nutrition, and (c) moderate exercise. By moderate, he means 90 minutes daily exercise such as walking, swimming, or light biking, that avoids excessive oxygen consumption leading to cascading free radicals. According to Lippman, “heavy aerobic exercise,” such as high-speed running, consumes up to eight times the amount of oxygen versus a resting state and generates huge cascades of free radicals.
Secondly, Dr. Lippman emphasizes avoiding “designer food” of the modern American food processing industry. Designer food is superbly engineered for its irresistibility as in the case of the Snickers Bar: We chew it, unhealthy sugar melts in our mouths, caramel captures the peanuts, fat seduces us, and the entire combination leaves the palate in a blissful state of ecstasy. Don’t be a designer-food victim. Instead, try consuming “Living Fuel” twice daily for optimal nutritional enhancement and health.
Thirdly, Dr. Lippman drives home the point that without hormone replacement therapy during aging, especially bioidentical thyroid (Armour), our lives become shortened by an estimated 43%.
In summary, Dr. Lippman says that anyone following his “one pill/one meal regimen” for one month, along with replacement of deficient hormones, nutrition, and moderate exercise, can expect superior blood test scores for markers such as C-reactive protein and sedimentation rate, achieving comparable values to those in their teens and twenties. “You will feel healthier and happier and your chronological age will begin to diverge from your biological age.” Lippman has followed this regimen since 1979 and maintains that he is biologically at least 15 to 20 years younger than his actual 64 years. And judging from his handsome and youthful photo, it seems to have worked in spades.
Fig. Four. Dr. Lippman at age 64.
References
1. Harman, . Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956; 11(3): 298–300.
2. Harman, D. A biologic clock: the mitochondria? J Amer Geriatr Soc. 1972; 20(4): 145–147.
3. Harman, D. Free radical Theory of Aging: the free radical diseases. AGE. 1984; 7:111-131.
4. DeGroot J. The AGE of the matrix: chemistry, consequence and cure. Curr Opin Pharmacol. 2004 Jun;4(3):301-5.
5. Harding JJ. Viewing molecular mechanisms of ageing through a lens. Ageing Res Rev. 2002 Jun;1(3):465-79.
6. Vlassara H. Advanced glycation in health and disease: role of the modern environment. Ann NY Acad Sci. 2005 Jun;1043:452-60.
7. Takeuchi M, Yamagishi S. TAGE (toxic AGEs) hypothesis in various chronic diseases. Med Hypotheses. 2004;63(3):449-52.
8. Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12(20):2293-315.
9. Hipkiss AR, Michaelis J, Syrris P. Non-enzymatic glycosylation of the dipeptide L-carnosine, a potential anti-protein-cross-linking agent. FEBS Lett. 1995 Aug 28;371(1):81-5.
10. Hipkiss AR. Carnosine, a protective, anti-ageing peptide? Int J Biochem Cell Biol. 1998 Aug;30(8):863-8.
11. Gallant S, Semyonova M, Yuneva M. Carnosine as a potential anti-senescence drug. Biochemistry (Mosc.). 2000 Jul;65(7):866-8.
12.. Valenzano DR, Terzibasi E, Genade T, et al. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006 Feb 7;16(3):296-300.
13. Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006 Nov 16;444(7117):337-42.
14. Lagouge M, Argmann C, Gerhart-Hines Z, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006 Dec 15;127(6):1109-22.
15. Borra MT, Smith BC , Denu JM. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005 Apr 29;280(17):17187-95.
16. Orallo F. Trans-resveratrol: a magical elixir of eternal youth? Curr Med Chem. 2008;15(19):1887-98.
17. Kaeberlein M, McDonagh T, Heltweg B, et al. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005 Apr 29;280(17):17038-45.
18. Guarente L, Picard F. Calorie restriction—the SIR2 connection. Cell. 2005 Feb 25;120(4):473-82.
19. Porcu M, Chiarugi A. The emerging therapeutic potential of sirtuin-interacting drugs: from cell death to lifespan extension. Trends Pharmacol Sci. 2005 Feb;26(2):94-103.
20. Ignatowicz E, Baer-Dubowska W. Resveratrol, a natural chemopreventive agent against degenerative diseases. Pol J Pharmacol. 2001 Nov;53(6):557-69.
21. Fremont L. Biological effects of resveratrol. Life Sci. 2000 Jan 14;66(8):663-73.
22. Dalessandri KM, Firestone GL, Fitch MD, Bradlow HL, Bjeldanes LF. Pilot study: effect of 3,3’-diindolylmethane supplements on urinary hormone metabolites in postmenopausal women with a history of early-stage breast cancer. Nutr Cancer. 2004;50(2):161-7.
23. Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42(1):1-9.
24. Wong GY, Bradlow L, Sepkovic D, et al. Dose-ranging study of indole-3-carbinol for breast cancer prevention. J Cell Biochem Suppl. 1997;28-29:111-6.
What our customers say…
Be the first to review this product, email your thoughts to us now.
