Peptide Bioregulators Promote Active and Functional Longevity
According to a 2012 United Nations estimate, over 300,000 people worldwide had reached their 100th birthday to become centenarians. Of these, a tiny percentage will go on to become supercentenarians, (110 years old and above) who characteristically experience relatively good health and an absence of age-related diseases until shortly before death at a very advanced age.
These enduring individuals are living proof that humans have the potential to live to 100, 110, or 120 years old, even beyond. What factors promote their exceptional longevity and why does 99.996 percent of the rest of the population age more quickly, lose functionality, develop diseases and die decades younger? This question is at the core of medical research aimed at determining the biological and genetic factors that result in age-related deterioration and developing effective strategies to counter them. Currently the therapeutic arsenal includes calorie restriction (and calorie restriction mimetics), antioxidants, glycation inhibitors, telomerase activators, stem cells and substances such as melatonin, carnosine, metformin, deprenyl and some others.
To add to this list, emerging research over the past four decades has uncovered the powerful life-extending potential of peptide bioregulators. These biologically active short chains of amino acids have the ability to repair genetic alterations occurring with age: they directly bind with portions of genes’ DNA to regulate and initiate processes that synthesize proteins, regenerate tissue, and restore functions of organs that have undergone age-related damage.
As a result of their rejuvenating properties, peptide bioregulators have been classified as “geroprotectors” (literally, “aging protectors,” i.e., drugs or substances that target the root causes of aging and degenerative diseases) and we’ll explore in this article their impressive longevity-promoting potential: in multiple studies, long-term treatment with various peptides has been shown to increase the lifespans of animals by up to 40 percent and significantly decrease mortality rates in aging humans while enhancing physiological function, physical performance and overall health.
The Aging Process
Aging, or senescence, is a complex biological process and trying to describe it invariably involves words with negative connotations: decline, decay, degeneration, death etc. One (very unpleasant) definition of aging is “the collection of changes that render human beings progressively more likely to die.” (1) A more detailed description is “a progressive loss of physiological integrity, leading to impaired function and increased vulnerability.” (2) So, to combine these, we can say that aging is associated with accumulating physiological impairments that lead to decreased function and increased risk of age-related diseases, (i.e., cancer, diabetes, cardiovascular disorders, neurodegenerative diseases, etc.) and of course mortality.
One of the lead researchers on peptide bioregulators and the genetic mechanisms of aging, Professor Vladimir Khavinson, (Director of the St. Petersburg Institute of Bioregulation and Gerontology, among other scientific positions), takes the definition a step further with this graphic description of aging which he describes as: “the gradual involution of tissues and development of organism malfunctioning [that] appears at end of the reproductive period and becomes more pronounced with age.” (3)

Professor Vladimir Khavinson is the former President of the European region of the International Association of Gerontology and Geriatrics; the main gerontologist of the Government of St. Petersburg and Director of the St. Petersburg Institute of Bioregulation and Gerontology.
The term involution denotes the shriveling, or shrinking of organs, or tissues that occurs over time due to reasons we’ll examine in the next section. Aging results in disturbances of the major regulatory systems in the body, (nervous, endocrine, and immune systems) and according to Khavinson, the atrophy of the thymus (immune system) and the pineal gland (neuroendocrine system) are the major manifestations of age-related decline. (4) Studies outlined below demonstrate that restoration of these and other organs results in dramatic improvement in physiological function and lengthening of lifespan in animals and humans.
Peptide Bioregulators Initiate a Crucial Process Altered by Age: Protein Synthesis
Previous research dating from as early as the 1960’s (initiated by Professor Vladimir Dilman of the same St. Petersburg based institute), had shown that small, low molecular weight regulatory peptides are involved the genetic transfer of biological information that leads to the synthesis of proteins. (3) Using this finding as the foundation for his research, and with the goal of regenerating tissue to restore the functions of age-damaged organs, Khavinson and his team began their work decades ago by devising a method for the isolation and purification of small peptides from extracts of various animal-sourced organs. (4)
These amino acid chains are endogenously produced in the cells of healthy tissues and play a regulatory role at the molecular and cellular levels that impacts the overall biochemical and physiological functioning of the particular organ in which they are active (hence the designation “peptide bioregulator”).
Each specific peptide interacts with specific sections of DNA, transferring information encoded in its amino acid sequence to regulate particular genes in a particular tissue. (4,5) Activation of these genes, (i.e., gene expression) stimulates protein synthesis, (4,6,7) the manufacture of proteins according to the encoded genetic information, a complex process indispensable to life. (See figure 1)
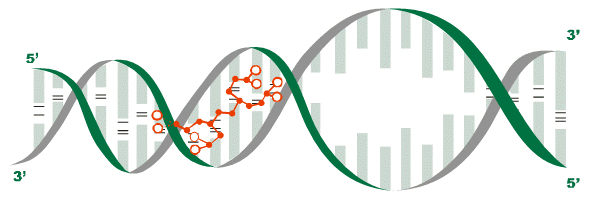
Figure 1: The interaction of a peptide bioregulator (as shown in red) with DNA initiates protein synthesis.
Depending on the specific genes expressed and the particular tissue involved, protein synthesis results in the assembly of thousands, even millions, of different substances necessary for overall physiological functioning, including enzymes, structural proteins such as collagen, hormones such as melatonin and insulin, antibodies, hemoglobin, etc.
Over time, however, changes in gene expression and impairments in endogenous peptide production result in decreased protein synthesis, leading to structural and functional deterioration of various organs, resulting in aging and age-related diseases (the “peptide theory of aging”). (4,5,7,8,9,11) Khavinson’s breakthrough discovery is that this degenerative cascade of aging can be reversed.
Peptide-Induced Regenerative and Longevity Effects in Animals
When administered to animals (and later to humans) Khavinson found that the peptide extracts he had isolated produced restorative effects in cells and tissues of the organ from which the particular peptide had originally been derived. (5) For instance, thymic peptides stimulated immunity (4,8) and pineal peptides induced the pineal gland to manufacture melatonin. (4,8, see figures 2 and 3 respectively).
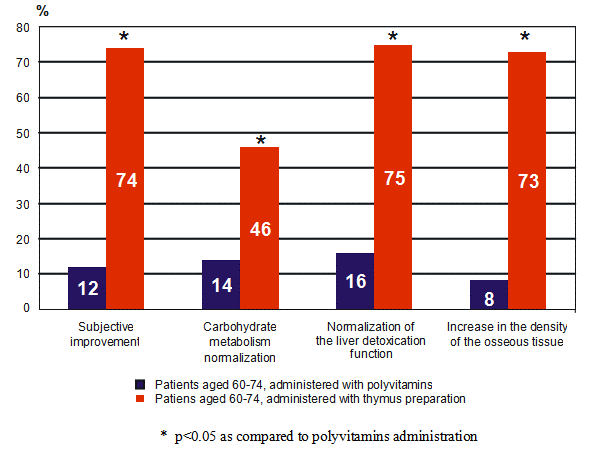
Figure 2: The effect of the thymus peptide bioregulator on metabolism in patients aged 60-74 years.
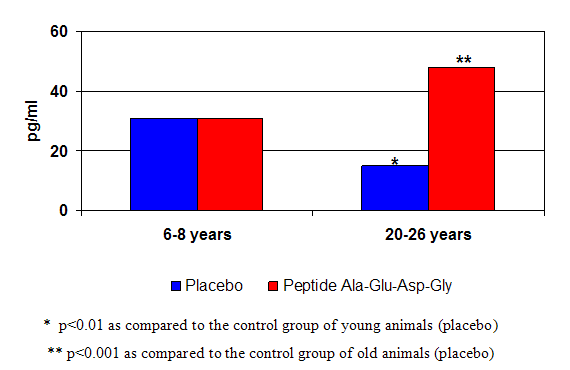
Figure 3: The pineal peptide bioregulator effect on melatonin production in monkeys of different age.
Trials using peptide preparations obtained from other organs, (i.e., retina, prostate, cerebral cortex, adrenal gland, liver, etc.) have all demonstrated beneficial effects on the condition and function of the respective gland, organ or tissues. (8) Amazingly, peptides isolated from organs of young animals were shown to trigger protein synthesis and restore the functions of aging, deteriorating organs in old animals. (4) For example, pineal peptides improved several aspects of age-related endocrine dysfunction by restoring reproductive function and fertility in old female rats that had undergone a process equivalent to menopause in women; (4) and increasing nightly melatonin production, normalizing daily cortisol secretion, decreasing glucose and insulin levels in aging rhesus monkeys. (11,12, see figure 4).
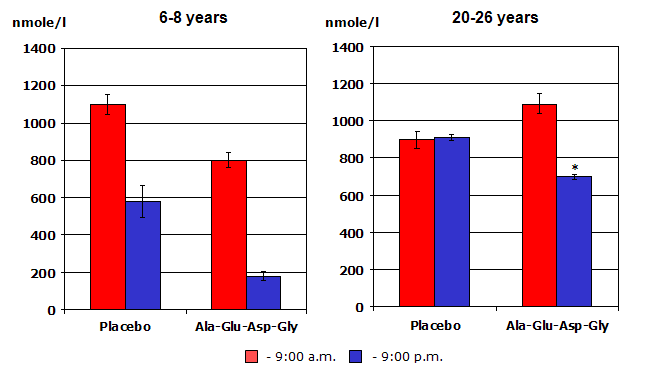
Figure 4: The adrenal peptide bioregulator effect on cortisol production in monkeys of different age, (in the morning and in the evening). Notice how the treated older animals’ cortisol production morning and evening now looks much closer to that of the young non-treated animals.
The results of Khavinson’s longevity experiments in animals were equally (if not more) compelling. In numerous studies over the course of three decades, long-term treatment with peptides isolated from thymus and pineal gland increased the average life span in drosophila (fruit flies), rats and mice by 20 to 40 percent (with some animals reaching their species maximum life span), slowed changes in biomarkers of aging and considerably suppressed tumor development. (4, see figure 5)
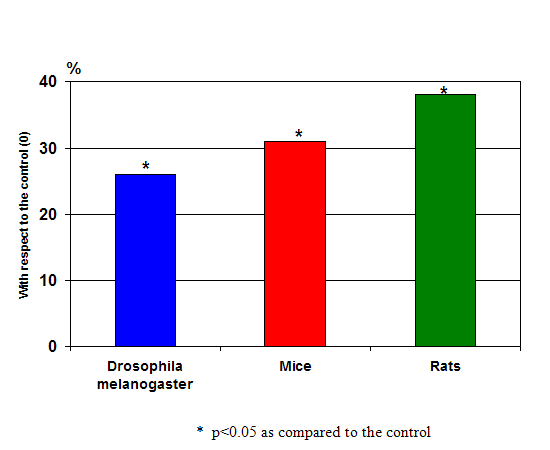
Figure 5: The increase in the average lifespan up to the specific limit after peptide preparations application, (mean results after 15 experiments) on fruit flies, mice and rats- all showing very significant increases in longevity over the controls.
These results have enormous implications for preventing cancer, retarding aging and extending life span in humans.
Another Peptide-Mediated Longevity Mechanism: Telomerase Activation
Besides stimulation of protein synthesis, Khavinson found that another genetic mechanism was operating to account for these impressive longevity results. In an experiment on cultured human lung fibroblasts (connective tissue cells), the addition of pineal peptide activated the telomerase gene and lengthened telomeres. Aging is associated with a decrease in the ability of cells to divide, (necessary for the construction and repair of tissues) due to the progressive shortening of structures called telomeres.
Telomeres are the protective “caps” at the ends of chromosomes and telomerase is an enzyme that adds repetitive DNA sequences to these “caps” each time a cell divides. (During cell division, telomeric portions of DNA are lost due to an inherent problem of incomplete end-replication. Telomerase ensures the complete copying of DNA repeats so that telomeres can maintain their length.) Most of the body’s cells, (excluding reproductive cells) can divide only a finite number of times, the so-called Hayflick limit, according to a programmed genetic clock that results in decreased telomerase production with age, leading to progressive shortening of telomeres, until cell division stops altogether.
This process, known as replicative senescence, ultimately leads to aging and age-related diseases. Khavinson found that the addition of pineal peptide to cultured human cells activated the telomerase gene to synthesize telomerase, resulting in lengthened telomeres and an increased number of cell divisions, overcoming the Hayflick limit. (4,13,14) This finding undoubtedly contributes to the extension of life span documented in the animal experiments.
With such remarkable results in cell culture and animal studies, Khavinson’s interest turned toward testing the peptides’ telomere lengthening effects in humans, those results are shown in figure 6.
| Age, years | Investigation | Telomere length (b.p.) | |||
| Peptide Preparations | |||||
| Epithalamin | Epitalon® | Endoluten® | |||
| 60-65 | Initial value |
9,32±0,82 (n=25) |
9,61±0,93 (n=19) |
9,43±1,12 (n=21) |
|
| After treatment | 10,83 ± 1,12* | 10,72 ± 1,21* | 10,62 ± 1,32* | ||
| 75-80 | Initial value |
7,33±0,81 (n=21) |
7,51±0,91 (n=17) |
7,63±0,98 (n=18) |
|
| After treatment | 8,73 ± 0,78* | 8,91 ± 1,11* | 8,66 ± 1,21* | ||
* – p< 0.05 as compared to initial value
Figure 6: These experiments show that all versions of the pineal peptide extend telomere length. These are blood samples taken from patients from the age of 60 to 80. Data on file: Laboratory of Biogerontology of the St. Petersburg Institute of Bioregulation and Gerontology.
As can be seen, in the study of 121 patients aged 60-80 years old, the use of the pineal peptide bioregulator produced an average increase of telomere length between 11% and 19%.
Biologic Reserve – the Capacity to go the Distance
According to Khavinson, and as his experiments in rats and mice demonstrate, the potential or maximum animal lifespan is approximately 30 to 40 percent higher than the actual average lifespan (4) for a particular species, so does this finding extend to humans as well? Well, for example, we know that humans can live to between 100 and 120 years old, (with the record for human longevity set at 122 years by Jeanne Calment of France), which is an extra 30 to 40 years above the average human lifespan of 80 years.
This capacity for three to four additional decades of life is what Khavinson calls the “biologic reserve.” (3) (See Figure 7.) Omitting factors such as predation, plagues, famine, wars, homicide, etc., why are animals and humans not reaching their species maximum lifespans? It is surmised it is because, that largely aging is a genetically determined and mediated process with inherent limitations.
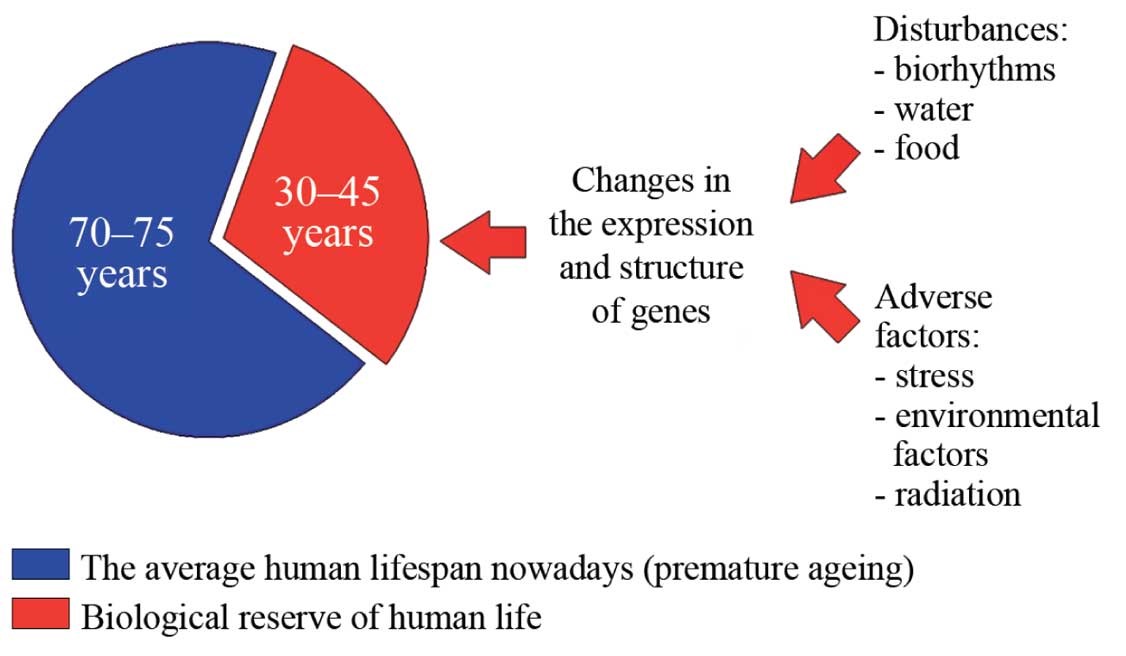
Figure 7: The average vs. the potential maximum human life span and the concept of the ‘biological reserve.’
For example, the telomere shortening and replicative senescence that we just discussed is one glitch in the genetic program of aging. Another is altered gene expression, the decline of endogenous peptide production and ergo impaired protein synthesis. As we saw earlier, tissue-specific peptides regulate the activity of particular genes in order to manufacture proteins: each peptide binds its amino acid sequence to a nucleotide sequence on a gene’s DNA (see figure 2) and sets into motion the transcriptional machinery that ultimately results in the synthesis of one of thousands of crucial proteins, (see figure 8).
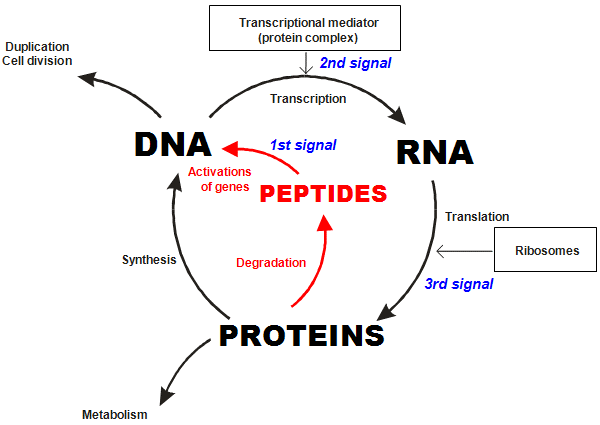
Figure 8: The role of peptide bioregulators in the cycle of DNA, RNA and protein biosynthesis.
Any flaw in this finely orchestrated and complex set of steps can lead to the downward spiral of organ deterioration, disease development and aging (i.e., a “biological deficit”) − one of the major reasons that animals and humans fall short of reaching their potential maximum longevity.
It should be mentioned that built-in genetic malfunctions are not the only culprits − negative environmental factors such as stressors (external and internal), radiation, pollution, pesticides and other toxins, inadequate nutrition, etc., can also affect biological mechanisms and hinder the regulation of protein synthesis, leading to accelerated or premature aging.
Summary
Delivery of the appropriate peptides can ameliorate or reverse these degenerative processes, essentially “restocking” the biological reserve. We can think of biological reserve as “supplementary life span potential” or the difference between the maximum number of years one could live under optimal physiological conditions when the genetic mechanisms of aging are slowed or reversed, such as with the use of peptide bioregulators and the average life span for the species when the aging program progresses unabated, which is shorter, at least in part due to the impact of the above mentioned limitations of the genetic aging program, as well as negative environmental factors.
Are there any examples relative to humans? Yes there are!
Human Studies with Peptide Bioregulators
After the experiments on drosophila, rats, mice and monkeys, the obvious and immediate question was whetherthe impressive results could be duplicated in humans. A slew of studies, many of them large-scale, were performed in the Soviet Union to find out, specifically with regard to improving physiological function, preventing and ameliorating diseases and enhancing longevity.
In one of these trials, seniors aged 60 to 74 years were treated with pineal peptide for 12 years, and older seniors aged 75 to 89 were treated with both pineal and thymus peptides for six years. Control groups for each age category received only vitamins. In this and other human trials, Khavinson calculated mortality rates, (the number of deaths in each of the control and treatment groups over a period of time) as an inverse indicator of longevity (i.e., a decreased mortality rate is equivalent to an increased survival rate, indicating that more people are staying alive longer). Unlike the animal experiments in which the lifespans of short-lived species could be easily measured, obviously, it was not practical or possible to conduct human studies over several decades to determine the ages at death of the longest-living people, so instead mortality rates were used.
The results showed that, compared to controls, those in the combined peptide treatment group demonstrated improved brain, immune, endocrine and cardiovascular function, as well as increased bone density, more youthful nocturnal melatonin levels and, as expected, lowered mortality rates, (see figure 9).
On this last point, in the 60 to 74 year old group receiving the pineal peptide bioregulator, the mortality rate was about half (22 percent) that of the control group (44 percent) over the course of 12 years.
Meanwhile, the older seniors aged 75 to 89 years receiving both thymus and pineal gland preparations for six years cut their mortality rate even further, to 33 percent, compared to 82 percent in their control group — a significant and remarkable improvement, especially in this older age group. (4)
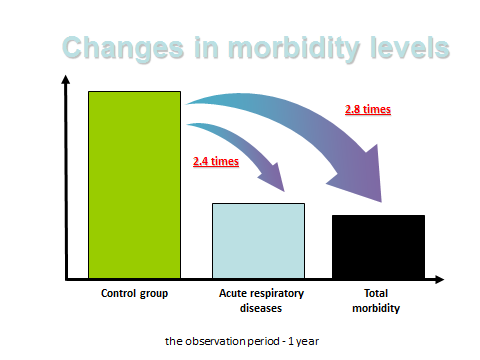
Figure 9: 450 factory workers treated with the peptide bioregulators for brain, pineal and thymus. The treated group has a 2.4 times less respiratory disease and 2.8 less times morbidity compared to 400 controls (15).
Khavinson also conducted several studies on the effects of peptide bioregulators on industrial workers in Russia who are often submitted to unsatisfactory conditions with negative health consequences, including premature aging. Some large studies were done on hundreds of employees of the automobile and electricity industries who exhibited physical improvements and protection against aging in response to treatment with various combinations of peptide bioregulators. The largest of these was on thousands of workers at Gazprom, a huge gas extraction and gas transportation company in Siberia. These employees are exposed to extreme environmental and work conditions that result in increased incidence of chronic diseases, premature aging and higher than average mortality rates.
Khavinson developed the “Program of Age-Related Pathology Prevention and Professional Longevity Extension” to counter these negative effects and restore health to these workers. Over 11,000 employees, (aged 35 to 60) received six oral peptides (immune, brain, blood vessels, bronchi, liver and cartilage) for 30 days. A control group of 3,000 workers received oral multivitamins for the same duration and both groups were monitored for one year.
The results showed that the peptide bioregulator treated group cut the frequency of acute respiratory disease by more than half and the overall incidence of disease by two-thirds, compared to the control group. In addition, they demonstrated improved work performance and attendance, reduced “biological age” (a measure of mental and physical capacity, as opposed to chronological age) and improved quality of life. (16, see figure 10)
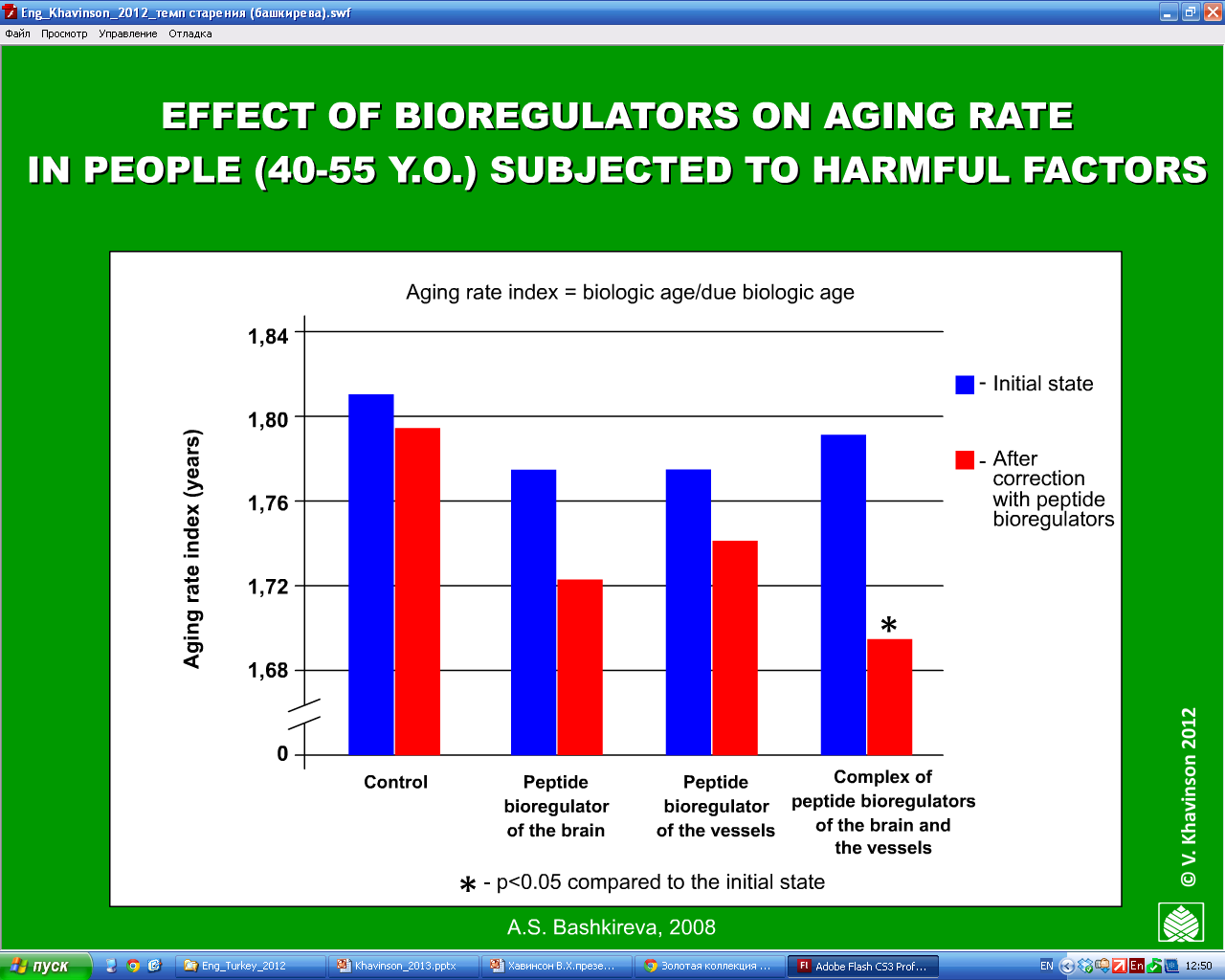
Figure 10: The effect after 1-year of peptide bioregulators on the rate of aging on 300 factory workers aged 40-55 years whom were under the influence of harmful factors, (compared to 200 controls who took multivitamins). Biological age measurements were improved in all the peptide bioregulator treated individuals.
All of these positive health outcomes over the course of the one year observation period reflect the geroprotective effects of peptide bioregulators in suppressing environmentally-induced premature aging and increasing biologic reserve in the thousands of workers studied.
Conclusion
We’ve covered a lot of territory in this article, so let’s recap the main points.
- Biologically active tissue-specific peptides correct age-related genetic malfunctions which would otherwise result in the typical degenerative process of aging: organ atrophy, loss of function, disease development, and shortened life span.
- Through the regulation of particular genes in particular tissues, peptides have the ability to reinitiate a process stalled by age, namely protein synthesis, leading to the repair and rebuilding of damaged tissues and organs and restoration of their main functions.
- When used in combination, their rejuvenating effects are not just organ-specific but system-wide, resulting in significant increases in life span in animals and decreased mortality rates in humans, along with lowered disease incidence, reduced biological age, and improved physiological function and physical performance, all of which indicate enhanced biological reserve.
- Through the peptide regulation of aging, humans now have the potential opportunity to add two or three (or perhaps more) decades of life to perhaps achieve the longevity of centenarians, or even supercentenarians, while remaining active, functional, vibrant and healthy. The studies highlight that the two most significant peptide bioregulators to date, in this respect are those emanating from the thymus and the pineal glands.
References
- www.senescence.info/aging_definition.html
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194-217.
- https://www.antiaging-systems.com/articles/peptide-regulation-of-aging/
- Anisimov V.N., Khavinson V.Kh. Peptide bioregulation of aging: results and prospects. Biogerontology. 2010;11:139-149.
- Khavinson V.Kh., Anisimov V.N. Peptide Regulation of Aging: 35-Year Research Experience. Bulletin of Experimental Biology and Medicine. 2009;148:94-98.
- Khavinson V.Kh., Kuznik B.I., Ryzhak G.A. Peptide Bioregulators: A New Class of Geroprotectors. Message 1: Results of Experimental Studies. Advances in Gerontology. 2013;3(3):225-235.
- Khavinson V.Kh., Lin’kova N.S., Trofimov A.V., Polyakova V.O., Sevost’yanova N.N., Kvetnoy I.M. Morphofunctional Fundamentals for Peptide Regulation of Aging. Biology Bulletin Reviews. 2011;1(4):390-394.
- Khavinson VKh. Peptides and Ageing. Neuro Endocrinol Lett. 2002;23 Suppl 3:11-144.
- Khavinson V.Kh., Solov’ev A.Yu., Zhilinskii D.V., Shataeva L.K., Vanyushin B.F. Epigenetic Aspects of Peptide-Mediated Regulation of Aging. Advances in Gerontology. 2012;2(4):277-286.
- Khavinson V.Kh., Tarnovskaya S.I., Linkova N.S., Pronyaeva V.E., Shataeva L.K., Yakutseni P.P. Short Cell-Penetrating Peptides: A Model of Interactions with Gene Promoter Sites. Bulletin of Experimental Biology and Medicine. 2013;154(3):403-408.
- Goncharova N.D., Lapin B.A., Khavinson V.Kh. Age-Associated Endocrine Dysfunctions and Approaches to Their Correction. Bulletin of Experimental Biology and Medicine. 2002;134(5):417-421.
- Goncharova N.D., Vengerin A.A., Khavinson V.Kh., Lapin B.A. Pineal peptides restore the age-related disturbances in hormonal functions of the pineal gland and the pancreas. Experimental Gerontology. 2005;40:51-57.
- Khavinson V.Kh., Bondarev I.E., Butyugov A.A. Epithalon Peptide Induces Telomerase Activity and Telomere Elongation in Human Somatic Cells. Bulletin of Experimental Biology and Medicine. 2003;135(6):590-592.
- Khavinson V.Kh, Bondarev I.E, Butyugov A.A., Smirnova T.D. Peptide Promotes Overcoming of the Division Limit in Human Somatic Cell. Bulletin of Experimental Biology and Medicine. 2004;137(5):613-616.
- Khavinson, V. Program of Age-Related Pathology Prevention and Professional Longevity Extension among “Gazprom” Personnel. Gerontology. 2001 July. 47(1): 453-454.
- Bashkireva A.S., Konovalov S.S. Prevention of accelerated aging of working in harmful conditions. Moscow 2001.