TRH Thyrotropin-releasing Hormone And Obesity
aInterbion Foundation for Basic Biomedical Research, Gordola, Switzerland
bFred Hutchinson Cancer Research Center, Seattle, WA, USA
Abstract
Adult adipose mice, high fat diet-fed (HFD) mice, anterior hypothalamus-lesioned obese mice and genetically obese mice, were injected daily with thyrotropin releasing hormone (TRH). The treatment provoked a mobilization of triglycerides in the peripheral blood, a decrease of leptin and a loss of body weight. The weight loss did not depend on TSH-mediated stimulation of thyroid hormone secretion with consequent metabolic hyperthyroidism. The levels of blood cholesterol was not affected or even suppressed. Even at a very high dosage TRH did not affect the obesity of genetically obese mice. The ubiquitous tripeptide TRH may thus constitute a key element in the hormone-controlled regulation of body weight and fat stores in the adult and aging body.
Introduction
Earlier studies have shown that TRH exerts thymus-protecting, immunoenhancing, antiviral, metabolic and lipid lowering effects in adult and old, aging mice [1, 2]. TRH antagonizes the thymolytic-immunosuppressive effects of corticosteroids via a non-thyroid mediated pathway [1, 2]. It was also shown that increasing doses of TRH do not significantly modify levels of thyroid hormones in peripheral blood of mice [1-5]. It has also been shown that prolonged TRH-tartrate administration does not produce hyperthyroidism in euthyroid cerebrovascular diseased patients [6, 7] and does not cause significant alterations of neuroendocrine balance [8]. It became thus clear that TRH, a ubiquitous and hylogenetically ancient peptide [9, 10], exerts effects in mammals which can rapidly correct aging-related metabolic dysfunctions [11]. In fact short-term treatment of aging mice with TRH by the intraperitoneal (ip) route rapidly normalizes levels of lipids to those of young animals [11] while chronic and combined, oral administration of melatonin and TRH corrects abnormally high triglyceride and cholesterol levels in old mice [5]. These basic observations suggested to investigate a possible activity of TRH in adult, adiposity-affected mice, in mice made obese by lesion of the anterior hypothalamic area (AHA), the so-called ‘thyrotropic region’, containing the highest concentration of TRH receptors and high affinity TRH binding sites, and in high fat diet (HFD)-fed mice [2, 10, 11]. The AHA is anatomically distinct from the ventromedial nucleus of the hypothalamus, the ablation of which also results in obesity [2]. The effect of ip and oral administration of TRH was tested also in genetically obese, non-diabetic mice.
Research Methods and Procedures
Animals
The animals used were inbred, male or female C57BL/6J and ob/ob, genetically obese, non diabetic C57BL/6 female mice. The animals were maintained in our fully air-conditioned animal rooms and given tap water and food pellets. Room temperature was 20-22 °C. Illumination was: 7 AM lights on; 7PM lights off.
Outbred CD1 female mice purchased from Charles River Animal Farm, Calco, Italy maintained at room temperature (18-22 °C) in non air-conditioned animal rooms with natural illumination and given tap water and food in pellets) and swine lard (high fat diet, HFD) ad libitum. All experiments shown in this report were performed in compliance with the Swiss Law for Animal Experimentation (Federal Law 455, 1978 and 455.1, 1981 and prescription 2.01 of February 3, 1994). Adult, lean mice are those young mice which reach adulthood with no adiposity to the age of one year. Adult, adiposity affected mice are those mice which start showing adiposity after the age of one year. Aging mice are those selected after the age of 18 months.
Anterior hypothalamic area (AHA) lesioning
The mice were lesioned at 2 months of age. The stereotaxis method adopted to produce a lesion of the AHA has been described in detail in a previous report [2]. The AHA-lesioned mice were selected on the basis of the obvious obesity ensuing from 2 to 4 months after the operation [12] and reaching peak values at 7-8 months after lesion of the AHA [2,12]. They were equally distributed in the experimental groups according to their body weight.
TRH
Synthetic TRH, tartrate salt (5-Oxo-L-prolyl-L-histidyl-L-prolinamide monotartrate monohydrate) certified 100 percent pure, was used.
Administration of TRH via the intraperitoneal route
When TRH was given by daily ip injection, it was diluted in bidistilled sterile water at the wished concentration and kept at 5°C until use within 6 hours. On the basis of previous studies [5], it was injected daily in the evening at 5-6 p.m. at the dose of 0.3 mg/kg b.w. in 0.2 ml volume/mouse.
Determination of TSH and thyroid hormones
Blood samples were collected separately from individual mice at 9 AM by using heparin-wet glass Pasteur pipettes. The blood was rapidly taken from the venous retroorbital plexus under halothane narcosis. The heparinized blood was centrifuged and the plasma samples were kept at -20°C until measurement of total thyroxine (T4), total triiodothyronine (T3) and TSH. The measurement was performed with the Architect system c8200 also used for routine clinical laboratory measurements.
Determination of lipids (cholesterol and triglycerides)
The same individual samples collected as described above were used for measurement of lipids. The measurement was performed with the Architect system c8200 also used for routine clinical laboratory measurements.
Determination of leptin
The same individual samples collected as described above were used for measurement of leptin by enzyme immunometric assay.
Determination of free fatty acid (FFA)
The same individual samples collected as described above were used for measurement of free fatty acid by enzyme colorimetric assay.
Food intake of TRH-treated mice
In one experiment we measured the food intake of groups of untreated and TRH-treated mice every 24 hours for periods of two weeks. The measurement of food intake was performed every day for each mouse housed in metabolic non-galvanized cages.
Statistical analysis
Results are expressed as mean ± SD. The significances between the means were assessed using paired Student’s t-test. Correlation was determined by linear regression analysis by the least square method. Differences were considered statistically significant when p< 0.05.
Results
TRH effect on body weight
As shown in Table 1, 20-day, i.p. daily administration of 0.3 mg/kg b.w. of TRH to adult, 9-month-old male C57BL/6 mice produced a significant loss of body weight, which was not visible in the control group (Table 1). When TRH was administered i.p. for 30 days in 12-month-old male C57BL/6 mice it produced a significant loss of body weight after 13 day of treatment, which was not visible in the control group. A small, non significant weight loss induced by the injection stress was observed also in the control group (Table 2a).
TABLE 1: Twenty-day, daily treatment with thyrotropin releasing hormone (TRH) reduces significantly body weight, produces mobilization of triglycerides and lowers levels of cholesterol in the blood of non-obese adult male mice.
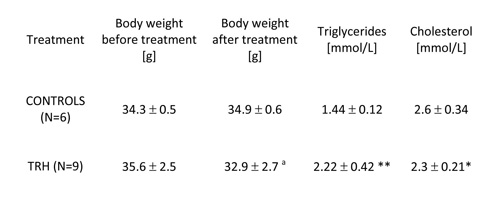
Mean ± SD, a p
TABLE 2a: 30-day daily treatment with thyrotropin releasing hormone (TRH) produces weight loss in 12 month-old C57BL/6 male mice. Thyroid hormones in peripheral blood are significantly decreased.

Mean ± SD, *p
TABLE 2b: 30-day daily treatment with thyrotropin releasing hormone (TRH) produces an early surge of triglycerides and lowers leptin levels in peripheral blood of 12 month-old C57BL/6 male mice.
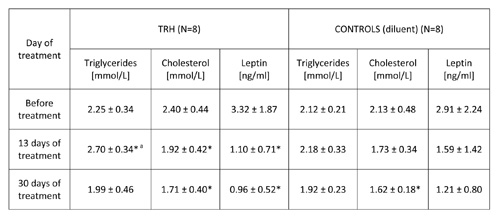
Mean ± SD, *p
TABLE 3: Chronic, 20-day daily treatment with thyrotropin releasing hormone (TRH) normalizes body weight, lowers blood levels of total T4, mobilizes triglycerides and lowers cholesterol in the blood of anterior hypothalamic area (AHA)-lesioned obese mice. The mice reacquire the original obesity after interruption of TRH administration.
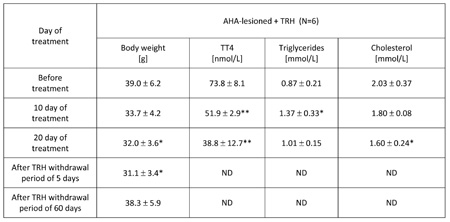
Mean ±SD, *p
As shown in Table 3, a 20-day daily treatment with 0.3 mg TRH/kg b.w. of obese AHA-lesioned, 7 month-old male mice produced a significant weight loss. After interruption of TRH treatment, the weight-normalized mice reacquired the original obesity within 60 days (Table 3). As shown in another experiment reported in Table 4 in which AHA-lesioned, control diluent-treated mice were included, a 48-day, daily treatment with TRH normalizes the body weight of female, 8 month-old AHA-lesioned obese mice, when compared to the diluent-treated, AHA-lesioned, obese control group. A small, non significant weight loss induced by the injection stress was observed also in the control group (Table 4).
TABLE 4: 48-day treatment with thyrotropin releasing hormone (TRH) lowers blood levels of total T4, produces mobilization of triglycerides and normalizes the body weight in anterior hypothalamic area (AHA)-lesioned obese mice.
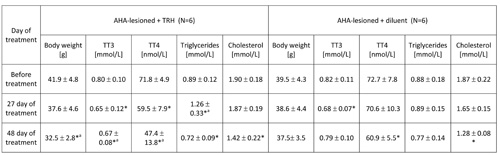
Mean ± SD, *p
As shown in Table 5, a 26-day, daily treatment with 0.3 mg/kg b.w. of TRH to obese, 5 month-old female CD1 outbreed, HFD-fed mice produced a progressive body weight loss, statistically significant after a withdrawal period of 2 days. After interruption of TRH treatment, the weight-normalized mice reacquired the original obesity within 30 days (Table 5).
TABLE 5: Daily injections of thyrotropin releasing hormone (TRH) in high fat diet fed (HFD) mice produces weight loss and a decreased food intake. A rapid regain of body weight occurs after TRH withdrawal.
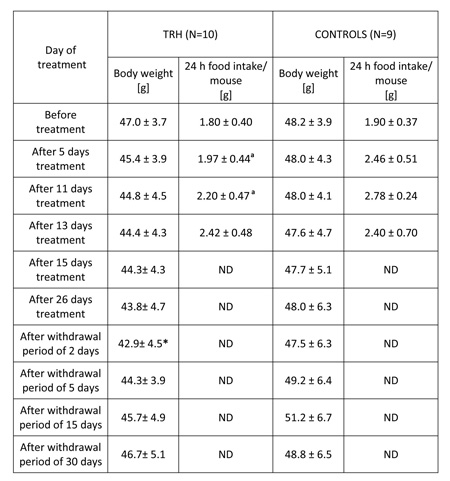
Mean ± SD, *p
Prolonged i.p. daily injection of daily doses of TRH (0.3 mg, 3 mg and 6 mg/ kg b.w.) in groups of 10, female, C57BL/6 ob/ob genetically obese non diabetic mice did not modify their obesity and their constant weight gain. Also when TRH was administered in their drinking water at the high dosage of 500 mg/ml, no modification of obesity could be observed. Therefore TRH is unable to modify obesity of genetically obese, non-diabetic mice (data not shown here).
TRH effects on lipid levels in peripheral blood of mice
As illustrated in Table 1, 20-day daily treatment with TRH, besides producing a significant loss of body weight, induced a mobilization of triglycerides and lowered cholesterol levels in peripheral blood of male, adult C57BL/6 mice. As shown in Table 2b, a daily treatment with 0.3 mg/kg b.w. of TRH produced a mobilization of triglycerides in peripheral blood of 12-month-old male C57BL/6 mice after 13 day of treatment, which was not visible in the control group. Treatment with TRH decreased cholesterol level. However this effect was visible also in the control group parallel to a minor decrease of body weight due to an obvious injection stress (Table 2a).
As seen in Table 3, a 20-day, treatment with one daily injection of 0.3 mg/kg b.w. of TRH produced a rapid mobilization of triglycerides and lowered cholesterol in the blood of AHA-lesioned, obese male C57BL/6 mice. When the experiment was repeated by using AHA-lesioned obese, female mice, the same mobilization of triglycerides in peripheral blood of the TRH-treated mice was observed, with decrease of cholesterol levels which however was visible also in the control group (Table 4).
As shown in Table 6, a 15-day, daily treatment with TRH produced, besides a decrease of body weight (Table 5), a simultaneous lowering of triglycerides and cholesterol in peripheral blood of HFD-fed obese mice.
TRH effects on total thyroxin (T4) and total triiodothyronin (T3) levels in peripheral blood of mice
As shown in Table 2a, prolonged daily TRH treatment in 12-month-old mice induced a significant decrease of T3 and T4 in peripheral blood. Tables 3 and 4 show that daily injection of TRH for 20 and 48 days in AHA-lesioned obese mice also lowers T3 and T4 levels when compared to control, diluent treated AHA-lesioned obese mice.
15-day, daily treatment in HFD-fed obese mice also showed that TRH produces a drop of T3 and T4 levels (Table 6). An enhancing effect of TRH on TSH, T3 and T4 was seen only when mice were acutely injected with TRH and blood was taken 2 hours after TRH injection (Table 7).
TABLE 6: Daily treatment with thyrotropin releasing hormone (TRH) induces a decrease of triglycerides and cholesterol levels, and a drop of leptin levels in peripheral blood of high-fat-diet-fed (HFD) mice. TRH treatment lowers thyroid hormone levels.
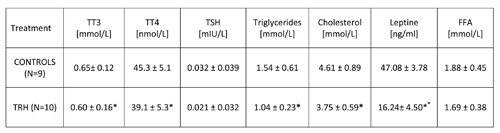
Mean ± SD, *p
TABLE 7: Acute effects of daily injections of thyrotropin releasing hormone (TRH) on thyroid function and lipid levels in peripheral blood of HFD adult female mice
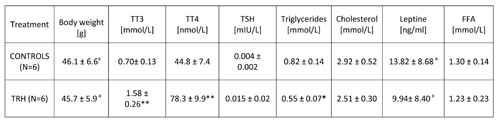
Mean ± SD, *p
TRH effects on leptin levels in peripheral blood of mice
Table 2b shows that a daily TRH treatment in 12-month-old male C57BL/6 mice produced a drop of leptin level which was parallel to the decrease of body weight (Table 2a). The same effect of TRH on reduction of leptins levels was seen in HFD-fed obese mice (Table 6). In fact, a positive correlation exists between leptin levels and body weight (r = 0.70, p<0.05) (Table 7).
Effects of TRH on levels of free fatty acid (FFA)
In all experiments, we could not observe any significant variation of FFA in both acute and prolonged daily treatment with TRH (Tables 6 and 7).
Food intake of TRH-treated HFD-fed mice
The daily morning measurement of food pellets and swine lard consumption of 26-day TRH-treated and untreated mice showed that the mice undergoing a daily treatment with TRH ate significantly less than control groups during the first 11 days. Food consumption of both groups was similar after that initial period (Table 5)
Discussion
The results reported above demonstrate a clear-cut slimming effect of a daily, exogenous TRH administration on the body weight of adipose adult C57BL/6 male mice, AHA-lesioned inbred C57BL/6 female mice and HFD-fed obese male and female out-bred albino mice. When the treatment is discontinued, the body weight of the originally adiposity-affected or clearly obese, TRH-treated mice return to the original adiposity or obesity values in the course of a few weeks.
As shown by measurement of total T4 and total T3 in the blood, and as observed in earlier work [1, 2, 5], TRH does not act by increasing levels of thyroid hormones (Tables 2a, 3 and 4). On the contrary, TRH produces a progressive diminution of T4 and T3 in peripheral blood (Tables 2a, 3 and 4). Most interesting, large amounts of triglycerides are mobilized in the peripheral blood shortly after initiation of TRH treatment of normal adult or obese mice (Tables 1, 2b, 3 and 4). Remarkably, this mobilization is not anticipated by liberation of free fatty acids (Tables 6 and 7) and takes place only at the time of treatment when the animals lose weight rapidly. Contrarily to what might be expected from the surge of triglycerides in their blood, TRH lowers the levels of cholesterol in the adult mice (Table 1). Cholesterol was significantly reduced in TRH-treated but also in the control groups of 12-month-old mice (Table 2b) and similar results are obtained in AHA- lesioned, obese mice (Table 4). This effect may reflect an injection-stress-induced weight loss and a parallel decrease of cholesterol, independent of treatment [13, 14].
The weight-reducing effects observed could be attributed to a loss of appetite and a lower food intake of the TRH-treated mice [15] (Table 5). In fact HFD-fed and TRH-treated mice consistently ate less than untreated mice at the time when their thyroid function was blunted (Table 6). The TRH lowering effect of thyroid function can be attributed to weight loss [16]. As expected, TRH profoundly lowered leptin levels in combination with weight loss (Table 2b and 6) [16-19].
Leptin, the product of ob gene, is secreted by adipocytes and has been shown to decrease food intake, decrease body weight, and increase energy expenditure through stimulation of thyrotropin-releasing hormone and activation of the sympathetic nervous system [20, 21]. Serum leptin levels correlate with body weight [20, 21] (Table 7). Futhermore, leptin acts centrally to decrease neuropeptide-Y (NPY) synthesis and NPY levels in the hypothalamic arcuate nucleus (ARC)–paraventricular nuclei (PVN) projection. NPY controls energy balance by stimulating feeding and inhibiting thermogenesis, especially under conditions of energy deficit [22]. Leptin levels rapidly decline with caloric restriction and weight loss. This decline is associated with adaptive physiological responses to starvation including increased appetite and decreased energy expenditure [23].The reduction in circulating levels of leptin serves as an important homeostasis signal to neurons of the hypothalamic arcuate nucleus that synthesizes NPY [24]. Furthermore, NPY administration suppresses circulating levels of thyroid hormones T3 and T4 and results in a normal or low TSH. These alterations were associated with a significant suppression of proTRH mRNA in the PVN [24].
These obesity-reducing effects of TRH cannot be attributed to central hypothalamic ‘satiety’ resulting into an induced restricted caloric diet effect [19] or to chronic, TRH-induced hyperthyroidism [6-8]. This effect of TRH was not reproducible in genetically leptin deficient [25] obese ob/ob C57BL/6 mice (data not shown here) in spite of prolonged administration of progressively higher doses of TRH by i.p. and oral route. We are unable to specify the cause of the failure of TRH in reversing the obese phenotype of the animals. However changes in food intake and energy expenditure such as body temperature and 02consumption were not measured. A central hypothalamic abnormality between genetic and acquired, diet-induced obesity, may be based on different mechanisms.
Our findings disclose a remarkable activity of exogenous TRH in the reduction and/or treatment of aging-related adiposity, diet-induced, and operationally induced hypothalamic obesity. At this stage of the research, no conclusion can be drawn on the mechanism of the effects observed. The weight loss can only temporarily depend on a diminished food intake (low calorie diet due to a decreased appetite, Table 5) or on a TRH-produced hyperthyroidism with consequent accelerated metabolic rate (Table 7). Both in naturally adipose adult mice, in HFD-fed, and in AHA-lesioned, obese mice, TRH can rapidly lower body weight. A rapid mobilization of triglycerides is visible in the peripheral blood a few days after initiation of TRH treatment, concomitantly with the rapid loss of body weight and decline of leptin levels. Apparently TRH produces rapid metabolic changes. TRH is in fact a physiological regulator of pituitary hormone secretion and hormonal regulation. TRH induces the ‘burst phase’ of acute hormone secretion by activation of protein kinase C via diacylglycerol and a simultaneous spike in calcium [26, 27]. This rapid ‘burst’ action of exogenous TRH on hormone synthesis and release seems to correct the metabolic derangements consequent to aging [5] and/or to replace endogenous TRH when the brain area in the hypothalamus, which is known to produce large amounts of TRH, is lesioned [2, 12]. However, the mechanism of this anti-obesity effect of TRH is largely unknown (28-30). It may be linked to feedback inhibition of insulin-producing beta cells in the Langerhans islets of the pancreas, which are known to produce and to secrete TRH [31-35]. The effects of TRH in the inhibition of amylase secretion in rats through a direct effect on acinar cells, on the enhancement of basal glucagon secretion and on the potentiation of glucose-stimulated insulin secretion, suggest that TRH may play a role in various pancreatic functions [31-35].
We cannot reduce the significance of the ubiquitous and multifunctional tripeptide TRH, possessing such an amazing variety of hormonal-interrelated effects [10, 11], without proposing a unifying concept, as illustrated in a number of experimental findings [1-5, 26-30]. We think that TRH is a key element for the general mechanism of operation of the pineal network [1-5, 41]. This peptide may be crucial in the mechanism by which both melatonin and pineal grafting postpone aging [3]. In fact, TRH is probably one of the basic mediators in the brain (pineal-hypothalamic-hypophyseal axis) and in peripheral endocrine glands (e.g. the b, insulin-producing cells in the pancreas). TRH may directly translate the light and temperature-mediated environmental stimuli into rapid energy-adapting biochemical processes which constantly monitor cell functions which relate to energy production, in particular those required for thermoregulation [5, 31]. We show here that this energy-monitoring action of TRH is not thyroid-mediated. In fact, leptin, melatonin and TRH follow a strict circadian periodicity [36, 37]. As a ‘rapid effector agent’ contained in the pineal gland [31, 38] and in the beta cells of the pancreas [31], clearly TRH provides to amplify hormonal activities via its proposed ‘burst action’ [26]. This hormone-amplifying burst action exerted by TRH on all cells and tissues needing rapid and ‘life saving’ activities such as the rapid release of insulin, is probably one of the mechanisms by which the ‘pineal clock’ maintains hormonal cyclicity and immunological functions [3, 39-44]. Aging-related visceral obesity which can be corrected by TRH is just one of the metabolic alterations accompanying the onset and progress of aging in the pineal gland [43-45].
Being TRH is a peptide with no known noxious side-effects even at high dosages [6-8, 46-48], clinical investigations on these effects of TRH for the correction of a ‘normal’ adiposity consequent to aging, or of pathological dietary obesity afflicting the affluent western society is feasible.
TRH may in fact be one of the novel peptides in the hypothalamus which regulate energy homoeostasis via adipocyte-derived hormones such as leptin [21, 23, 47-49].
References
- Pierpaoli W and Yi CX. The involvement of pineal gland and melatonin in immunity and aging. I. Thymus-mediated, immunoreconstituting and antiviral activity of thyrotropin releasing hormone (TRH). J Neuroimmunol 27:99-109 (1990).
- Lesnikov VA, Korneva E.A, Dall’Ara A and Pierpaoli W. The involvement of pineal gland and melatonin in immunity and aging. II. Thyrotropin releasing hormone and melatonin forestall involution and promote reconstitution of the thymus in anterior hypothalamic area (AHA)-lesioned mice. Int J. Neurosci 68:123-131 (1993).
- Pierpaoli W and Regelson W. The pineal control of aging. The effect of melatonin and pineal grafting on aging mice. Proc Natl Acad Sci. USA 91:787-791 (1994).
- Pierpaoli W. The pineal aging clock. Evidence, models and an approach to aging-delaying strategies. In: Aging, Immunity and Infections (Edited by Powers, D.C., Morley, J.E. and Coe, R.M.), pp. 166-175. Springer Publishing Company, New York (1994).
- Pierpaoli W, Bulian D, Bulian G and Kistler, G. Thyrotropin releasing hormone (TRH) accelerates and enhances the aging-postponing effects of melatonin. J Anti-Aging Med 4:343-348 (1999).
- Monzani F, Pucci E, Fierabracci P, Nebbiai, G, Coli A, Caraccio N et al. Long-term intramuscular administration of thyrotropin-releasing hormone tartrate in patients with cerebrovascular disease: effects on the pituitary-thyroid axis. Horm Res 35:146-150 (1991).
- Custro N, Scafidi V, Costanzo G and Corsello FP. Changes in blood levels of the pituitary-thyroid axis observed during therapy with protirelin ++ in hospitalized euthyroid patients with cerebrovascular disease. Minerva Med 82:453-457 (1991).
- Caraccio N, Monzani F, Casolaro A, Pucci E, Luisi M, Caraccio N et al. Tolerance of long-term protirelin tartrate treatment. Recent Prog Med 86:226-230 (1995).
- Pierpaoli W and Lesnikov VA. The pineal aging clock. Evidence, models, mechanisms, interventions. Ann NY Acad Sci 719:461-473 (1994).
- Jackson IMD. Thyrotropin-releasing hormone. New Engl J Med 306:145-155 (1982).
- Griffith EC. Thyrotropin releasing hormone: endocrine and central effects. Psychoneuroendocrinol 10:225-235 (1985).
- Pierpaoli W and Lesnikov VA. Theoretical considerations on the nature of the pineal « ageing clock ». Gerontol 43:20-25 (1997).
- Kiortsis DN, Tzotzas T, Girald P, Bruckert E, Beucler I, Valsamides S et al. Changes in lipoprotein(a) levels and hormonal correlation during a weight reduction program. Nutr Metab Cardiovasc Dis 11:153 157 (2001).
- Guanche Garcell H, Beatriz Torres M, Martinez Quesada C, Gutierrez Garcia F, Canizares Gomez N, et al. Effect of weight reduction on lipids and lipoprotein (a) serum levels. Med Clin (Barc) 30; 119: 730-731 (2002)
- Steward CA, Horan TL, Schuhler S, Bennett GW and Ebling FJ. Central administration of thyrotropin releasing hormone (TRH) and related peptides inhibits feeding behavior in the Siberian hamster. Neuroreport 15:687-691 (2003).
- Reinehr T and Andler W. Thyroid hormones before and after weight loss in obesity. Arch Dis Child. 87:320-323 (2002).
- Ahrèn B, Mansson S, Gingerich RL and Havel PJ. Regulation of plasma leptin in mice: influence of age, high-fat diet, and fasting. AM J Physiol 273:113-120 (1997).
- Komorowski J, Jankiewicz-Wika J and Stepien H. Effects of Gn-RH, TRH, and CRF administration on plasma leptin levels in lean and obese women. Neuropeptides. 34:89-97 (2000).
- Garcia SI, Landra MS, Porto PI, Alvarez AL. Schuman, M., Finkielman, S. & Pirola, C.J. Thyrotropin-releasing hormone decreases leptin and mediates the leptin-induced pressor effect. Hypertension 39:491-495 (2002).
- Flier JS, Harris,M and Hollenberg AN. Leptin, nutrition, and the thyroid: the why, the wherefore, and the wiring. J Clin Invest 105:859-861 (2000).
- Korner J and Arone LJ. The emerging science of body weight regulation and its impact on obesity treatment. J Clin Invest 111:565-570 (2003).
- Wang Q, Bing C, Al-barazanji K, Mossakowasaka DE, Wang XM, McBay DL et al. Interactions between leptin and hypothalamic neuropeptide Y neurons in control of food intake and energy homeostasis in the rat. Diabetes 46:335-341 (1997).
- Kershaw EE and Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrin Metab 89:2548-2556 (2004).
- Fekete C, Kelly J, Mihàly E, Sarkar S, Rand WM, Lègràdi G et al. Neuropeptide Y has a central inhibitory action on the hypothalamic-pituitary-thyroid axis. Endocrinol 142:2606-2613 (2001).
- Hyogo H, S Roy S, B Paigen B, and Cohen DE. Leptin promotes biliary cholesterol elimination during weight loss in ob/ob mice by regulating the enterohepatic circulation of bile salts. J Biol Chem 277:34117-34124 (2002).
- Albert PR and Tashjian AH Jr. Dual actions of phorbol esters on cytosolic free Ca2+ concentrations and reconstitution with ionomycin of acute thyrotropin-releasing hormone responses. J Biol Chem 260:8746-8759 (1985).
- Kruskal BA, Keith CH and Maxfield FR. Thyrotropin-releasing hormone-induced changes in intracellular [Ca²+] measured by microspectrofluorometry on individual quin2- loaded cells. J Cell Biol 99: 1167-1172 (1984)
- Chiamolera MI and Wondisford FE. Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism Mini-review, Endocrinology 150:1091-1096 (2008)
- Hollenberg AN. The role of the thyrotropin-releasing hormone (TRH) neuron as a metabolic sensor Thyroid 16: 131-139 (2008)
- Kamath J, Yarbrough GG, Prange Jr AJ and Winokur A. The thyrotropin-releasing hormone (TRH)-immune system homeostatic hypothesis Pharm & Therap 121:20-28 (2009)
- Martino E, Seo H, Lernmark A and Refetoff S. Ontogenetic patterns of thyrotropin-releasing hormone-like material in rat hypothalamus, pancreas and retina: selective effect of light deprivation. Proc Natl Acad Sci USA 77:4345-4348 (1980).
- Benicky J and Strbak V. Glucose stimulates and insulin inhibits release of pancreatic TRH in vivo. Eur J Endocrinol 142:60-65 (2000).
- Yano N and Luo L. Effect of thyrotropin hormone (TRH) on gene expression in rat pancreas: approach by microarray hybridization. JOP 5:193-204 (2004).
- Luo LG, Jackson I, Thyrotropin releasing hormone (TRH) may preserve pancreatic islet function: potential role in the treatment of diabetes mellitus. Acta Biomed 78, suppl. 1: 216-221 (2007)
- Luo LG, Jackson I, Thyrotropin releasing hormone (TRH) may preserve pancreatic islet function: potential role in the treatment of diabetes mellitus. Acta Biomed 78, suppl. 1: 216-221 (2007)
- Mazzacolli G, Giuliani A, Carughi S, De Cata A, Puzzolante F, La Viola et al. The hypothalamic-pituitary axis and melatonin in humans: Possibile interactions in the control of body temperature. Neuroendocrinol Let 25:368-372 (2004).
- Alonso-Vale MI, Andreotti S, Peres SB, Anhe GF, das Neves Borges-Silva C, Neto JC, Lima FB. Melatonin enhances leptin expression by rat adipocytes in the presence of insulin. Am J Physiol Metab 288:E805-12 (2004).
- Jackson IMD: Evolutionary significance of the phylogenetic distribution of the mammalian hypothalamic releasing hormones. Fed Proc 40:2545-2552 (1981)
- Pierpaoli W: The pineal aging clock: an approach to age-delaying strategies, in Powers] DC, Morley JE, Coe RM: Aging, Immunity and Infection. New York: Springer Publishing Company 166-175 (1994).
- Pierpaoli W, Dall’Ara A, Pedrinis E and Regelson W: The pineal control of aging. The effects of melatonin and pineal grafting on the survival of older mice. Ann NY Acad Sci 621:291-449 (1991).
- Pierpaoli W, Bulian D, Dall’Ara A, Marchetti B, Gallo F, Morale MC et al. Circadian melatonin and young-to-old pineal grafting postpone aging and maintain juvenile conditions of reproductive functions in mice and rats. Exp Gerontol 32:587-602 (1997).
- Mocchegiani E., Bulian D., Santarelli L, Tibaldi A, Muzzioli M, Lesnikov, V. A et al. The zinc pool is involved in the immune-reconstituting effect of melatonin in pinealectomized mice. J Pharmacol and Exp Therapeut 277:1200-1208 (1996).Mocchegiani E, Bulian, D, Santarelli L, Tibaldi A, Muzzioli M, Pierpaoli W et al. The immuno-reconstituting effect of melatonin or pineal grafting and its relation to zinc pool in aging mice J Neuroimmunol 53:189-201 (1994).
- Pierpaoli W and Bulian D. The pineal aging and death program I. Grafting of old pineals in young mice accelerates their aging. J Anti-Aging Med 4:31 –37 (2001)
- Wolden-Hanson T, Mitton DR, McCants RL, Yellow SM, Wilkinson CW, Matsumoto AM, et al. Daily melatonin administration to middle-aged male rats suppresses body we
